The epithelium in idiopathic pulmonary fibrosis: breaking the barrier
- PMID: 24454287
- PMCID: PMC3887273
- DOI: 10.3389/fphar.2013.00173
The epithelium in idiopathic pulmonary fibrosis: breaking the barrier
Abstract
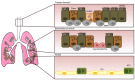
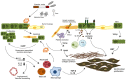
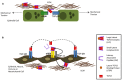
Idiopathic pulmonary fibrosis is a progressive disease of unknown etiology characterized by a dysregulated wound healing response that leads to fatal accumulation of fibroblasts and extracellular matrix (ECM) in the lung, which compromises tissue architecture and lung function capacity. Injury to type II alveolar epithelial cells is thought to be the key event for the initiation of the disease, and so far both genetic factors, such as mutations in telomerase and MUC5B genes as well as environmental components, like cigarette smoking, exposure to asbestos and viral infections have been implicated as potential initiating triggers. The injured epithelium then enters a state of senescence-associated secretory phenotype whereby it produces both pro-inflammatory and pro-fibrotic factors that contribute to the wound healing process in the lung. Immune cells, like macrophages and neutrophils as well as activated myofibroblasts then perpetuate this cascade of epithelial cell apoptosis and proliferation by release of pro-fibrotic transforming growth factor beta and continuous deposition of ECM stiffens the basement membrane, altogether having a deleterious impact on epithelial cell function. In this review, we describe the role of the epithelium as both a physical and immunological barrier between environment and self in the homeostatic versus diseased lung and explore the potential mechanisms of epithelial cell injury and the impact of loss of epithelial cell permeability and function on cytokine production, inflammation, and myofibroblast activation in the fibrotic lung.
Keywords: TGF-β; apoptosis; epithelium; fibroblasts; idiopathic pulmonary fibrosis.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

