Transgenic strategies to confer resistance against viruses in rice plants
- PMID: 24454308
- PMCID: PMC3888933
- DOI: 10.3389/fmicb.2013.00409
Transgenic strategies to confer resistance against viruses in rice plants
Abstract
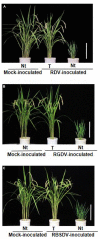
Rice (Oryza sativa L.) is cultivated in more than 100 countries and supports nearly half of the world's population. Developing efficient methods to control rice viruses is thus an urgent necessity because viruses cause serious losses in rice yield. Most rice viruses are transmitted by insect vectors, notably planthoppers and leafhoppers. Viruliferous insect vectors can disperse their viruses over relatively long distances, and eradication of the viruses is very difficult once they become widespread. Exploitation of natural genetic sources of resistance is one of the most effective approaches to protect crops from virus infection; however, only a few naturally occurring rice genes confer resistance against rice viruses. Many investigators are using genetic engineering of rice plants as a potential strategy to control viral diseases. Using viral genes to confer pathogen-derived resistance against crops is a well-established procedure, and the expression of various viral gene products has proved to be effective in preventing or reducing infection by various plant viruses since the 1990s. RNA interference (RNAi), also known as RNA silencing, is one of the most efficient methods to confer resistance against plant viruses on their respective crops. In this article, we review the recent progress, mainly conducted by our research group, in transgenic strategies to confer resistance against tenuiviruses and reoviruses in rice plants. Our findings also illustrate that not all RNAi constructs against viral RNAs are equally effective in preventing virus infection and that it is important to identify the viral "Achilles' heel" gene to target for RNAi attack when engineering plants.
Keywords: RNA interference; Reoviridae; Tenuivirus; forage rice cultivar; transgenic rice.
Figures



References
-
- Attoui H., Mertens P. P. C., Becnel J., Belaganahalli S., Bergoin M., Brussaard C. P., et al. (2011). “Family reoviridae,” in Virus Taxonomy: Classification and Nomenclature, Ninth Report of the International Commitee on Taxonomy of Viruses eds King A. M. Q., Adams M. J., Carstens E. B., Lefkowitz E. J. (San Diego, CA: Academic Press; ) 541–637
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

