Structural Features of the Peptide Homologous to 6-25 Fragment of Influenza A PB1 Protein
- PMID: 24454411
- PMCID: PMC3886529
- DOI: 10.1155/2013/370832
Structural Features of the Peptide Homologous to 6-25 Fragment of Influenza A PB1 Protein
Abstract
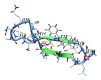
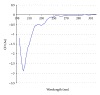
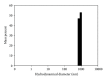
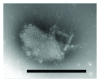
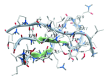
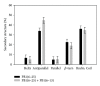
A mirror-symmetry motif was discovered in the N-terminus of the influenza virus PB1 protein. Structure of peptide comprised of the corresponding part of PB1 (amino acid residues 6-25) was investigated by circular dichroism and in silico modeling. We found that peptide PB1 (6-25) in solution assumes beta-hairpin conformation. A truncated peptide PB1 (6-13), containing only half of the mirror-symmetry motif, appeared to stabilize the beta-structure of the original peptide and, at high concentrations, was capable of reacting with peptide to form insoluble aggregates in vitro. Ability of PB1 (6-13) peptide to interact with the N-terminal domain of PB1 protein makes it a potential antiviral agent that inhibits PA-PB1 complex formation by affecting PB1 N-terminus structure.
Figures

References
-
- Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discovery Today. 2010;15(1-2):40–56. - PubMed
-
- He X, Zhou J, Bartlam M, et al. Crystal structure of the polymerase PAC-PB1N complex from an avian influenza H5N1 virus. Nature. 2008;454(7208):1123–1126. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

