Synthesis of five- and six-membered cyclic organic peroxides: Key transformations into peroxide ring-retaining products
- PMID: 24454562
- PMCID: PMC3896255
- DOI: 10.3762/bjoc.10.6
Synthesis of five- and six-membered cyclic organic peroxides: Key transformations into peroxide ring-retaining products
Abstract
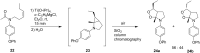
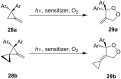
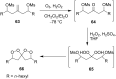
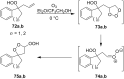
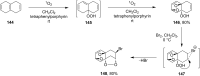
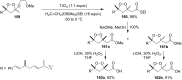
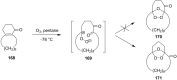
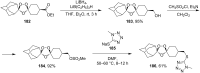
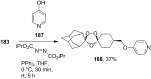
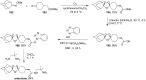
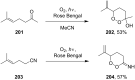
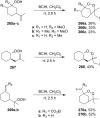
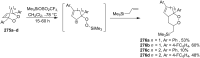
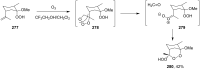
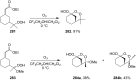
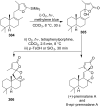
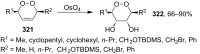
The present review describes the current status of synthetic five and six-membered cyclic peroxides such as 1,2-dioxolanes, 1,2,4-trioxolanes (ozonides), 1,2-dioxanes, 1,2-dioxenes, 1,2,4-trioxanes, and 1,2,4,5-tetraoxanes. The literature from 2000 onwards is surveyed to provide an update on synthesis of cyclic peroxides. The indicated period of time is, on the whole, characterized by the development of new efficient and scale-up methods for the preparation of these cyclic compounds. It was shown that cyclic peroxides remain unchanged throughout the course of a wide range of fundamental organic reactions. Due to these properties, the molecular structures can be greatly modified to give peroxide ring-retaining products. The chemistry of cyclic peroxides has attracted considerable attention, because these compounds are used in medicine for the design of antimalarial, antihelminthic, and antitumor agents.
Keywords: 1,2,4,5-tetraoxanes; 1,2,4-trioxanes; 1,2,4-trioxolanes; 1,2-dioxanes; 1,2-dioxenes; 1,2-dioxolanes; cyclic peroxides; ozonides.
Figures





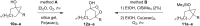
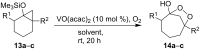
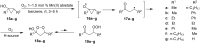


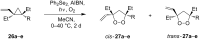




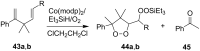

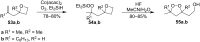





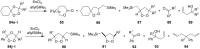


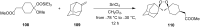
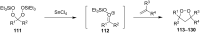



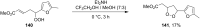






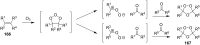

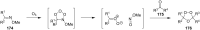



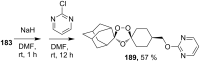


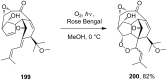
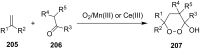


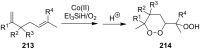
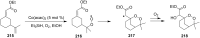

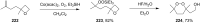
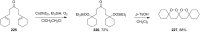



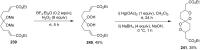
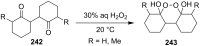

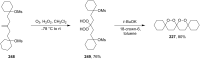



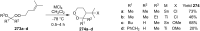
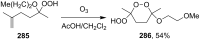
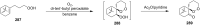










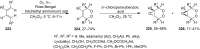




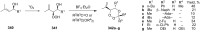
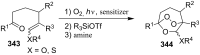



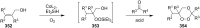
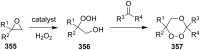
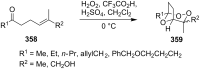


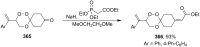





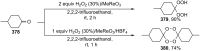


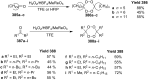
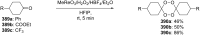















Similar articles
-
Catalyst Development for the Synthesis of Ozonides and Tetraoxanes Under Heterogeneous Conditions: Disclosure of an Unprecedented Class of Fungicides for Agricultural Application.Chemistry. 2020 Apr 9;26(21):4734-4751. doi: 10.1002/chem.201904555. Epub 2020 Feb 19. Chemistry. 2020. PMID: 31774931
-
Synthetic peroxides as potent antimalarials. News and views.Curr Top Med Chem. 2012;12(5):373-99. doi: 10.2174/156802612799362940. Curr Top Med Chem. 2012. PMID: 22242847 Review.
-
Exploration of artemisinin derivatives and synthetic peroxides in antimalarial drug discovery research.Eur J Med Chem. 2021 Mar 5;213:113193. doi: 10.1016/j.ejmech.2021.113193. Epub 2021 Jan 18. Eur J Med Chem. 2021. PMID: 33508479 Review.
-
Approach for the preparation of various classes of peroxides based on the reaction of triketones with H2O2: first examples of ozonide rearrangements.Chemistry. 2014 Aug 4;20(32):10160-9. doi: 10.1002/chem.201402594. Epub 2014 Jul 2. Chemistry. 2014. PMID: 24989116
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
Cited by
-
Cobalt-Catalyzed Intramolecular Silylperoxidation of Unsaturated Diisopropylsilyl Ethers.J Org Chem. 2019 Jun 21;84(12):7564-7574. doi: 10.1021/acs.joc.9b00642. Epub 2019 May 30. J Org Chem. 2019. PMID: 31046281 Free PMC article.
-
Electrocatalysis with Molecular Transition-Metal Complexes for Reductive Organic Synthesis.JACS Au. 2022 May 31;2(6):1266-1289. doi: 10.1021/jacsau.2c00031. eCollection 2022 Jun 27. JACS Au. 2022. PMID: 35783173 Free PMC article. Review.
-
Advanced optical waveguide design via encapsulation of 2,4,6-triphenylpyrylium chloride in oxide glasses.Nanoscale. 2025 Aug 15. doi: 10.1039/d5nr02213d. Online ahead of print. Nanoscale. 2025. PMID: 40814860 Free PMC article.
-
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches.Beilstein J Org Chem. 2020 May 6;16:917-955. doi: 10.3762/bjoc.16.83. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32461773 Free PMC article. Review.
-
Triethylamine-Catalyzed Cyclization of Unsaturated Hydroperoxides in the Presence of Triethylammonium Hydrochloride: A Synthesis of 1,2-Dioxanes.Org Lett. 2025 Mar 7;27(9):2037-2041. doi: 10.1021/acs.orglett.4c04629. Epub 2025 Feb 26. Org Lett. 2025. PMID: 40009755 Free PMC article.
References
-
- McCullough K J, Nojima M. Curr Org Chem. 2001;5:601. doi: 10.2174/1385272013375346. - DOI
-
- Korshin E E, Bachi M D. Synthesis of cyclic peroxides. In: Rappoport Z, editor. The Chemistry of Peroxides. 2, Part 1. Chichester: John Wiley & Sons, Ltd; 2006. p. 189. - DOI
-
- Slack R D, Jacobine A M, Posner G H. Med Chem Commun. 2012;3:281. doi: 10.1039/c2md00277a. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
