The regioselective synthesis of spirooxindolo pyrrolidines and pyrrolizidines via three-component reactions of acrylamides and aroylacrylic acids with isatins and α-amino acids
- PMID: 24454564
- PMCID: PMC3896290
- DOI: 10.3762/bjoc.10.8
The regioselective synthesis of spirooxindolo pyrrolidines and pyrrolizidines via three-component reactions of acrylamides and aroylacrylic acids with isatins and α-amino acids
Abstract
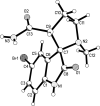
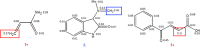
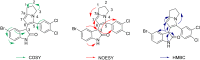
The regioselective three-component condensation of azomethine ylides derived from isatins and α-amino acids with acrylamides or aroylacrylic acids as dipolarophiles has been realized through a one-pot 1,3-dipolar cycloaddition protocol. Decarboxylation of 2'-aroyl-2-oxo-1,1',2,2',5',6',7',7a'-octahydrospiro[indole-3,3'-pyrrolizine]-1'-carboxylic acids is accompanied by cyclative rearrangement with formation of dihydropyrrolizinyl indolones.
Keywords: acrylamides; aroylacrylic acids; azomethine ylide; cyclative rearrangement; cycloaddition; multicomponent; spirooxindoles.
Figures







References
-
- Jossang A, Jossang P, Hadi H, Sevenet A T, Bodo B. J Org Chem. 1991;56:6527–6530. doi: 10.1021/jo00023a016. - DOI
-
- Jones K, Wilkinson J. J Chem Soc, Chem Commun. 1992:1767–1769. doi: 10.1039/C39920001767. - DOI
-
- Palmisano G, Annuziata R, Papeo G, Sisti M. Tetrahedron: Asymmetry. 1996;7:1–4. doi: 10.1016/0957-4166(95)00406-8. - DOI
-
- James M N G, Williams G J B. Can J Chem. 1972;50:2407–2412. doi: 10.1139/v72-386. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
