PDGF, NT-3 and IGF-2 in combination induced transdifferentiation of muscle-derived stem cells into Schwann cell-like cells
- PMID: 24454677
- PMCID: PMC3891637
- DOI: 10.1371/journal.pone.0073402
PDGF, NT-3 and IGF-2 in combination induced transdifferentiation of muscle-derived stem cells into Schwann cell-like cells
Abstract
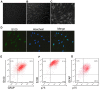
Muscle-derived stem cells (MDSCs) are multipotent stem cells with a remarkable long-term self-renewal and regeneration capacity. Here, we show that postnatal MDSCs could be transdifferentiated into Schwann cell-like cells upon the combined treatment of three neurotrophic factors (PDGF, NT-3 and IGF-2). The transdifferentiation of MDSCs was initially induced by Schwann cell (SC) conditioned medium. MDSCs adopted a spindle-like morphology similar to SCs after the transdifferentiation. Immunocytochemistry and immunoblot showed clearly that the SC markers S100, GFAP and p75 were expressed highly only after the transdifferentiation. Flow cytometry assay showed that the portion of S100 expressed cells was more than 60 percent and over one fourth of the transdifferentiated cells expressed all the three SC markers, indicating an efficient transdifferentiation. We then tested neurotrophic factors in the conditioned medium and found it was PDGF, NT-3 and IGF-2 in combination that conducted the transdifferentiation. Our findings demonstrate that it is possible to use specific neurotrophic factors to transdifferentiate MDSCs into Schwann cell-like cells, which might be therapeutically useful for clinical applications.
Conflict of interest statement
Figures



References
-
- Bunge RP (1994) The role of the Schwann cell in trophic support and regeneration. J Neurol 242: 19–21. - PubMed
-
- Ide C (1996) Peripheral nerve regeneration. Neurosci Res 25: 101–21. - PubMed
-
- Jessen KR, Mirsky R (1999) Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci 22: 402–10. - PubMed
-
- Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G (2001) Retroviral labelling of Schwann cells: In vitro charactarisation and in vivo transplantation to improve peripheral nerve regeneration. Glia 34: 8–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

