In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates
- PMID: 24454709
- PMCID: PMC3891645
- DOI: 10.1371/journal.pone.0083865
In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates
Abstract
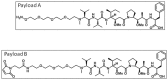
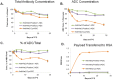
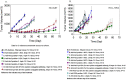
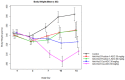
Antibody drug conjugates (ADCs) are monoclonal antibodies designed to deliver a cytotoxic drug selectively to antigen expressing cells. Several components of an ADC including the selection of the antibody, the linker, the cytotoxic drug payload and the site of attachment used to attach the drug to the antibody are critical to the activity and development of the ADC. The cytotoxic drugs or payloads used to make ADCs are typically conjugated to the antibody through cysteine or lysine residues. This results in ADCs that have a heterogeneous number of drugs per antibody. The number of drugs per antibody commonly referred to as the drug to antibody ratio (DAR), can vary between 0 and 8 drugs for a IgG1 antibody. Antibodies with 0 drugs are ineffective and compete with the ADC for binding to the antigen expressing cells. Antibodies with 8 drugs per antibody have reduced in vivo stability, which may contribute to non target related toxicities. In these studies we incorporated a non-natural amino acid, para acetyl phenylalanine, at two unique sites within an antibody against Her2/neu. We covalently attached a cytotoxic drug to these sites to form an ADC which contains two drugs per antibody. We report the results from the first direct preclinical comparison of a site specific non-natural amino acid anti-Her2 ADC and a cysteine conjugated anti-Her2 ADC. We report that the site specific non-natural amino acid anti-Her2 ADCs have superior in vitro serum stability and preclinical toxicology profile in rats as compared to the cysteine conjugated anti-Her2 ADCs. We also demonstrate that the site specific non-natural amino acid anti-Her2 ADCs maintain their in vitro potency and in vivo efficacy against Her2 expressing human tumor cell lines. Our data suggests that site specific non-natural amino acid ADCs may have a superior therapeutic window than cysteine conjugated ADCs.
Conflict of interest statement
Figures



Similar articles
-
Antibody-Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs.Mol Pharm. 2015 Nov 2;12(11):3986-98. doi: 10.1021/acs.molpharmaceut.5b00432. Epub 2015 Oct 2. Mol Pharm. 2015. PMID: 26393951 Free PMC article.
-
Efficient Preparation of Site-Specific Antibody-Drug Conjugates Using Cysteine Insertion.Mol Pharm. 2017 May 1;14(5):1501-1516. doi: 10.1021/acs.molpharmaceut.6b00995. Epub 2017 Mar 16. Mol Pharm. 2017. PMID: 28245132
-
Potent antitumor activity of anti-HER2 antibody-topoisomerase I inhibitor conjugate based on self-immolative dendritic dimeric-linker.J Control Release. 2024 Mar;367:148-157. doi: 10.1016/j.jconrel.2024.01.025. Epub 2024 Jan 25. J Control Release. 2024. PMID: 38228272
-
Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985).Eur J Med Chem. 2019 Dec 1;183:111682. doi: 10.1016/j.ejmech.2019.111682. Epub 2019 Sep 6. Eur J Med Chem. 2019. PMID: 31563805 Review.
-
Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions.Breast Cancer Res. 2021 Aug 11;23(1):84. doi: 10.1186/s13058-021-01459-y. Breast Cancer Res. 2021. PMID: 34380530 Free PMC article. Review.
Cited by
-
Design and characterization of homogenous antibody-drug conjugates with a drug-to-antibody ratio of one prepared using an engineered antibody and a dual-maleimide pyrrolobenzodiazepine dimer.MAbs. 2019 Apr;11(3):500-515. doi: 10.1080/19420862.2019.1578611. Epub 2019 Mar 5. MAbs. 2019. PMID: 30835621 Free PMC article.
-
Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes.Bioconjug Chem. 2014 Jul 16;25(7):1331-41. doi: 10.1021/bc500189z. Epub 2014 Jun 23. Bioconjug Chem. 2014. PMID: 24924618 Free PMC article.
-
Peptide Therapeutics: Unveiling the Potential against Cancer-A Journey through 1989.Cancers (Basel). 2024 Mar 2;16(5):1032. doi: 10.3390/cancers16051032. Cancers (Basel). 2024. PMID: 38473389 Free PMC article. Review.
-
Multiformat T-cell-engaging bispecific antibodies targeting human breast cancers.Angew Chem Int Ed Engl. 2015 Jun 8;54(24):7022-7. doi: 10.1002/anie.201500799. Epub 2015 Apr 27. Angew Chem Int Ed Engl. 2015. PMID: 25919418 Free PMC article.
-
Key metrics to expanding the pipeline of successful antibody-drug conjugates.Trends Pharmacol Sci. 2021 Oct;42(10):803-812. doi: 10.1016/j.tips.2021.07.005. Epub 2021 Aug 26. Trends Pharmacol Sci. 2021. PMID: 34456094 Free PMC article. Review.
References
-
- Goldmacher VS, Kovtun YV (2011) Antibody-drug conjugates: using monoclonal antibodies for delivery of cytotoxic payloads to cancer cells. Ther Deliv 2: 397–416. - PubMed
-
- Meden H, Kuhn W (1997) Overexpression of the oncogene c-erbB-2 (HER2/neu) in ovarian cancer: a new prognostic factor. Eur J Obstet Gynecol Reprod Biol 71: 173–179. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, et al. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182. - PubMed
-
- Papazisis KT, Habeshaw T, Miles DW (2004) Safety and efficacy of the combination of trastuzumab with docetaxel for HER2-positive women with advanced breast cancer. A review of the existing clinical trials and results of the expanded access programme in the UK. Int J Clin Pract 58: 581–586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

