HPTN 071 (PopART): a cluster-randomized trial of the population impact of an HIV combination prevention intervention including universal testing and treatment: mathematical model
- PMID: 24454728
- PMCID: PMC3893126
- DOI: 10.1371/journal.pone.0084511
HPTN 071 (PopART): a cluster-randomized trial of the population impact of an HIV combination prevention intervention including universal testing and treatment: mathematical model
Abstract
Background: The HPTN 052 trial confirmed that antiretroviral therapy (ART) can nearly eliminate HIV transmission from successfully treated HIV-infected individuals within couples. Here, we present the mathematical modeling used to inform the design and monitoring of a new trial aiming to test whether widespread provision of ART is feasible and can substantially reduce population-level HIV incidence.
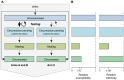
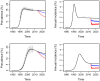
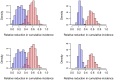
Methods and findings: The HPTN 071 (PopART) trial is a three-arm cluster-randomized trial of 21 large population clusters in Zambia and South Africa, starting in 2013. A combination prevention package including home-based voluntary testing and counseling, and ART for HIV positive individuals, will be delivered in arms A and B, with ART offered universally in arm A and according to national guidelines in arm B. Arm C will be the control arm. The primary endpoint is the cumulative three-year HIV incidence. We developed a mathematical model of heterosexual HIV transmission, informed by recent data on HIV-1 natural history. We focused on realistically modeling the intervention package. Parameters were calibrated to data previously collected in these communities and national surveillance data. We predict that, if targets are reached, HIV incidence over three years will drop by >60% in arm A and >25% in arm B, relative to arm C. The considerable uncertainty in the predicted reduction in incidence justifies the need for a trial. The main drivers of this uncertainty are possible community-level behavioral changes associated with the intervention, uptake of testing and treatment, as well as ART retention and adherence.
Conclusions: The HPTN 071 (PopART) trial intervention could reduce HIV population-level incidence by >60% over three years. This intervention could serve as a paradigm for national or supra-national implementation. Our analysis highlights the role mathematical modeling can play in trial development and monitoring, and more widely in evaluating the impact of treatment as prevention.
Conflict of interest statement
Figures

References
-
- Attia S, Egger M, Muller M, Zwahlen M, Low N (2009) Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 23: 1397–1404. - PubMed
-
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG (2009) Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373: 48–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

