The effect of brain-derived neurotrophic factor on periodontal furcation defects
- PMID: 24454754
- PMCID: PMC3891769
- DOI: 10.1371/journal.pone.0084845
The effect of brain-derived neurotrophic factor on periodontal furcation defects
Abstract
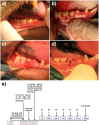
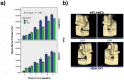
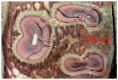
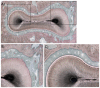
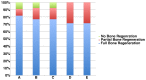
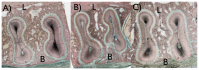
This study aimed to observe the regenerative effect of brain-derived neurotrophic factor (BDNF) in a non-human primate furcation defect model. Class II furcation defects were created in the first and second molars of 8 non-human primates to simulate a clinical situation. The defect was filled with either, Group A: BDNF (500 µg/ml) in high-molecular weight-hyaluronic acid (HMW-HA), Group B: BDNF (50 µg/ml) in HMW-HA, Group C: HMW-HA acid only, Group D: empty defect, or Group E: BDNF (500 µg/ml) in saline. The healing status for all groups was observed at different time-points with micro computed tomography. The animals were euthanized after 11 weeks, and the tooth-bone specimens were subjected to histologic processing. The results showed that all groups seemed to successfully regenerate the alveolar buccal bone, however, only Group A regenerated the entire periodontal tissue, i.e., alveolar bone, cementum and periodontal ligament. It is suggested that the use of BDNF in combination with a scaffold such as the hyaluronic acid in periodontal furcation defects may be an effective treatment option.
Conflict of interest statement
Figures



References
-
- Lindsay RM, Thoenen H, Barde YA (1985) Placode and neural crest-derived sensory neurons are responsive at early developmental stages to brain-derived neurotrophic factor. Dev Biol 112: 319–328. - PubMed
-
- Kubo T, Nonomura T, Enokido Y, Hatanaka H (1995) Brain-derived neurotrophic factor (BDNF) can prevent apoptosis of rat cerebellar granule neurons in culture. Brain Res Dev Brain Res 85: 249–258. - PubMed
-
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, et al. (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306: 487–491. - PubMed
-
- Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10: 209–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

