Olives and olive oil are sources of electrophilic fatty acid nitroalkenes
- PMID: 24454759
- PMCID: PMC3891761
- DOI: 10.1371/journal.pone.0084884
Olives and olive oil are sources of electrophilic fatty acid nitroalkenes
Abstract
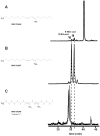
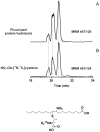
Extra virgin olive oil (EVOO) and olives, key sources of unsaturated fatty acids in the Mediterranean diet, provide health benefits to humans. Nitric oxide (•NO) and nitrite (NO2 (-))-dependent reactions of unsaturated fatty acids yield electrophilic nitroalkene derivatives (NO2-FA) that manifest salutary pleiotropic cell signaling responses in mammals. Herein, the endogenous presence of NO2-FA in both EVOO and fresh olives was demonstrated by mass spectrometry. The electrophilic nature of these species was affirmed by the detection of significant levels of protein cysteine adducts of nitro-oleic acid (NO2-OA-cysteine) in fresh olives, especially in the peel. Further nitration of EVOO by NO2 (-) under acidic gastric digestive conditions revealed that human consumption of olive lipids will produce additional nitro-conjugated linoleic acid (NO2-cLA) and nitro-oleic acid (NO2-OA). The presence of free and protein-adducted NO2-FA in both mammalian and plant lipids further affirm a role for these species as signaling mediators. Since NO2-FA instigate adaptive anti-inflammatory gene expression and metabolic responses, these redox-derived metabolites may contribute to the cardiovascular benefits associated with the Mediterranean diet.
Conflict of interest statement
Figures



References
-
- Frankel EN (2011) Nutritional and biological properties of extra virgin olive oil. J Agric Food Chem 59: 785–792. - PubMed
-
- Romani A, Lapucci C, Cantini C, Ieri F, Mulinacci N, et al. (2007) Evolution of minor polar compounds and antioxidant capacity during storage of bottled extra virgin olive oil. J Agric Food Chem 55: 1315–1320. - PubMed
-
- Catharino RR, Haddad R, Cabrini LG, Cunha IB, Sawaya AC, et al. (2005) Characterization of vegetable oils by electrospray ionization mass spectrometry fingerprinting: classification, quality, adulteration, and aging. Anal Chem 77: 7429–7433. - PubMed
-
- Rastrelli L, Passi S, Ippolito F, Vacca G, De Simone F (2002) Rate of degradation of alpha-tocopherol, squalene, phenolics, and polyunsaturated fatty acids in olive oil during different storage conditions. J Agric Food Chem 50: 5566–5570. - PubMed
-
- Cert A, Moreda W, Perez-Camino MC (2000) Chromatographic analysis of minor constituents in vegetable oils. J Chromatogr A 881: 131–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL132550/HL/NHLBI NIH HHS/United States
- R01-HL64937/HL/NHLBI NIH HHS/United States
- P01-HL103455/HL/NHLBI NIH HHS/United States
- P30-DK072506/DK/NIDDK NIH HHS/United States
- P01 HL103455/HL/NHLBI NIH HHS/United States
- R37 HL058115/HL/NHLBI NIH HHS/United States
- R01-HL058115/HL/NHLBI NIH HHS/United States
- R01 HL058115/HL/NHLBI NIH HHS/United States
- R01 AT006822-01/AT/NCCIH NIH HHS/United States
- P30 DK072506/DK/NIDDK NIH HHS/United States
- R01 AT006822/AT/NCCIH NIH HHS/United States
- R01 HL064937/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

