Toxicity of functional nano-micro zinc oxide tetrapods: impact of cell culture conditions, cellular age and material properties
- PMID: 24454775
- PMCID: PMC3890288
- DOI: 10.1371/journal.pone.0084983
Toxicity of functional nano-micro zinc oxide tetrapods: impact of cell culture conditions, cellular age and material properties
Abstract
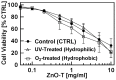
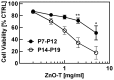
With increasing production and applications of nanostructured zinc oxide, e.g., for biomedical and consumer products, the question of safety is getting more and more important. Different morphologies of zinc oxide structures have been synthesized and accordingly investigated. In this study, we have particularly focused on nano-micro ZnO tetrapods (ZnO-T), because their large scale fabrication has been made possible by a newly introduced flame transport synthesis approach which will probably lead to several new applications. Moreover, ZnO-T provide a completely different morphology then classical spherical ZnO nanoparticles. To get a better understanding of parameters that affect the interactions between ZnO-T and mammalian cells, and thus their biocompatibility, we have examined the impact of cell culture conditions as well as of material properties on cytotoxicity. Our results demonstrate that the cell density of fibroblasts in culture along with their age, i.e., the number of preceding cell divisions, strongly affect the cytotoxic potency of ZnO-T. Concerning the material properties, the toxic potency of ZnO-T is found to be significantly lower than that of spherical ZnO nanoparticles. Furthermore, the morphology of the ZnO-T influenced cellular toxicity in contrast to surface charges modified by UV illumination or O2 treatment and to the material age. Finally, we have observed that direct contact between tetrapods and cells increases their toxicity compared to transwell culture models which allow only an indirect effect via released zinc ions. The results reveal several parameters that can be of importance for the assessment of ZnO-T toxicity in cell cultures and for particle development.
Conflict of interest statement
Figures








References
-
- Kathawala MH, Xiong S, Richards M, Ng KW, George S, et al. (2013) Emerging in-vitro models for safety screening of high-volume production nanomaterials under environmentally relevant exposure conditions. Small 9: 1504–1520. - PubMed
-
- Hussain SM, Braydich-Stolle LK, Schrand AM, Murdock RC, Yu KO, et al. (2009) Toxicity evaluation for safe use of nanomaterials: recent achievements and technical challenges. Adv. Mater. 21: 1549–1559.
-
- Han Z, Levchenko I, Kumar S, Yajadda M, Yick S, et al. (2011) Plasma nanofabrication and nanomaterials safety. J. Phys. D: Appl. Phys. 44: 174019.
-
- Landsiedel R, Ma-Hock L, Kroll A, Hahn D, Schnekenburger J, et al. (2010) Testing metal-oxide nanomaterials for human safety. Adv. Mater. 22: 2601–2627. - PubMed
-
- Kocbek P, Teska, Kreft ME, Kristl J (2010) Toxicological aspects of long-term treatment of keratinocytes with ZnO and TiO2 nanoparticles. Small 6: 1908–1917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

