Transmembrane and coiled-coil domain family 1 is a novel protein of the endoplasmic reticulum
- PMID: 24454821
- PMCID: PMC3891740
- DOI: 10.1371/journal.pone.0085206
Transmembrane and coiled-coil domain family 1 is a novel protein of the endoplasmic reticulum
Abstract
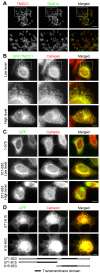
The endoplasmic reticulum (ER) is a continuous membrane network in eukaryotic cells comprising the nuclear envelope, the rough ER, and the smooth ER. The ER has multiple critical functions and a characteristic structure. In this study, we identified a new protein of the ER, TMCC1 (transmembrane and coiled-coil domain family 1). The TMCC family consists of at least 3 putative proteins (TMCC1-3) that are conserved from nematode to human. We show that TMCC1 is an ER protein that is expressed in diverse human cell lines. TMCC1 contains 2 adjacent transmembrane domains near the C-terminus, in addition to coiled-coil domains. TMCC1 was targeted to the rough ER through the transmembrane domains, whereas the N-terminal region and C-terminal tail of TMCC1 were found to reside in the cytoplasm. Moreover, the cytosolic region of TMCC1 formed homo- or hetero-dimers or oligomers with other TMCC proteins and interacted with ribosomal proteins. Notably, overexpression of TMCC1 or its transmembrane domains caused defects in ER morphology. Our results suggest roles of TMCC1 in ER organization.
Conflict of interest statement
Figures






Similar articles
-
A Novel Class of ER Membrane Proteins Regulates ER-Associated Endosome Fission.Cell. 2018 Sep 20;175(1):254-265.e14. doi: 10.1016/j.cell.2018.08.030. Epub 2018 Sep 13. Cell. 2018. PMID: 30220460 Free PMC article.
-
TMCC3 localizes at the three-way junctions for the proper tubular network of the endoplasmic reticulum.Biochem J. 2019 Nov 15;476(21):3241-3260. doi: 10.1042/BCJ20190359. Biochem J. 2019. PMID: 31696206
-
Targeting of a tail-anchored protein to endoplasmic reticulum and mitochondrial outer membrane by independent but competing pathways.Mol Biol Cell. 2001 Aug;12(8):2482-96. doi: 10.1091/mbc.12.8.2482. Mol Biol Cell. 2001. PMID: 11514630 Free PMC article.
-
Biochemical properties and cellular localisation of STIM proteins.Cell Calcium. 2007 Aug;42(2):123-32. doi: 10.1016/j.ceca.2007.02.006. Epub 2007 Mar 26. Cell Calcium. 2007. PMID: 17382385 Review.
-
Protein transport into the human endoplasmic reticulum.J Mol Biol. 2015 Mar 27;427(6 Pt A):1159-75. doi: 10.1016/j.jmb.2014.06.011. Epub 2014 Jun 23. J Mol Biol. 2015. PMID: 24968227 Review.
Cited by
-
Investigating the Genetic Links Between Immune Cell Profiles and Bladder Cancer: A Multidisciplinary Bioinformatics Approach.Biomedicines. 2025 May 15;13(5):1203. doi: 10.3390/biomedicines13051203. Biomedicines. 2025. PMID: 40427030 Free PMC article.
-
Spatial re-organization of myogenic regulatory sequences temporally controls gene expression.Nucleic Acids Res. 2015 Feb 27;43(4):2008-21. doi: 10.1093/nar/gkv046. Epub 2015 Feb 4. Nucleic Acids Res. 2015. PMID: 25653159 Free PMC article.
-
Unique transcriptional signatures of sleep loss across independently evolved cavefish populations.J Exp Zool B Mol Dev Evol. 2020 Nov;334(7-8):497-510. doi: 10.1002/jez.b.22949. Epub 2020 Apr 29. J Exp Zool B Mol Dev Evol. 2020. PMID: 32351033 Free PMC article.
-
Expression and characterization of transmembrane and coiled-coil domain family 3.BMB Rep. 2016 Nov;49(11):629-634. doi: 10.5483/bmbrep.2016.49.11.151. BMB Rep. 2016. PMID: 27697108 Free PMC article.
-
Transmembrane and coiled-coil domain family 3 (TMCC3) regulates breast cancer stem cell and AKT activation.Oncogene. 2021 Apr;40(16):2858-2871. doi: 10.1038/s41388-021-01729-1. Epub 2021 Mar 19. Oncogene. 2021. PMID: 33742122 Free PMC article.
References
-
- Dudek J, Lang S, Schorr S, Linxweiler J, Greiner M, et al. (2013) Analysis of protein translocation into the endoplasmic reticulum of human cells. Methods Mol Biol 1033: 285–299. - PubMed
-
- Matlack KE, Mothes W, Rapoport TA (1998) Protein translocation: tunnel vision. Cell 92: 381–390. - PubMed
-
- Ma Y, Hendershot LM (2001) The unfolding tale of the unfolded protein response. Cell 107: 827–830. - PubMed
-
- Hong W (1998) Protein transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Sci 111 ( Pt 19): 2831–2839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

