Role of receptor activity modifying protein 1 in function of the calcium sensing receptor in the human TT thyroid carcinoma cell line
- PMID: 24454825
- PMCID: PMC3890319
- DOI: 10.1371/journal.pone.0085237
Role of receptor activity modifying protein 1 in function of the calcium sensing receptor in the human TT thyroid carcinoma cell line
Abstract
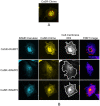
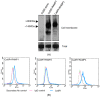
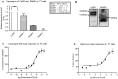
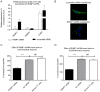
The Calcium Sensing Receptor (CaSR) plays a role in calcium homeostasis by sensing minute changes in serum Ca(2+) and modulating secretion of calciotropic hormones. It has been shown in transfected cells that accessory proteins known as Receptor Activity Modifying Proteins (RAMPs), specifically RAMPs 1 and 3, are required for cell-surface trafficking of the CaSR. These effects have only been demonstrated in transfected cells, so their physiological relevance is unclear. Here we explored CaSR/RAMP interactions in detail, and showed that in thyroid human carcinoma cells, RAMP1 is required for trafficking of the CaSR. Furthermore, we show that normal RAMP1 function is required for intracellular responses to ligands. Specifically, to confirm earlier studies with tagged constructs, and to provide the additional benefit of quantitative stoichiometric analysis, we used fluorescence resonance energy transfer to show equal abilities of RAMP1 and 3 to chaperone CaSR to the cell surface, though RAMP3 interacted more efficiently with the receptor. Furthermore, a higher fraction of RAMP3 than RAMP1 was observed in CaSR-complexes on the cell-surface, suggesting different ratios of RAMPs to CaSR. In order to determine relevance of these findings in an endogenous expression system we assessed the effect of RAMP1 siRNA knock-down in medullary thyroid carcinoma TT cells, (which express RAMP1, but not RAMP3 constitutively) and measured a significant 50% attenuation of signalling in response to CaSR ligands Cinacalcet and neomycin. Blockade of RAMP1 using specific antibodies induced a concentration-dependent reduction in CaSR-mediated signalling in response to Cinacalcet in TT cells, suggesting a novel functional role for RAMP1 in regulation of CaSR signalling in addition to its known role in receptor trafficking. These data provide evidence that RAMPs traffic the CaSR as higher-level oligomers and play a role in CaSR signalling even after cell surface localisation has occurred.
Conflict of interest statement
Figures


References
-
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, et al. (1993) Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580. - PubMed
-
- Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, et al. (1995) Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology 136: 5202–5211. - PubMed
-
- Lombardi G, Di Somma C, Rubino M, Faggiano A, Vuolo L, et al. (2011) The roles of parathyroid hormone in bone remodeling: prospects for novel therapeutics. Journal of endocrinological investigation 34: 18–22. - PubMed
-
- Kraenzlin ME, Meier C (2011) Parathyroid hormone analogues in the treatment of osteoporosis. Nature reviews Endocrinology 7: 647–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

