Novel method to load multiple genes onto a mammalian artificial chromosome
- PMID: 24454889
- PMCID: PMC3893256
- DOI: 10.1371/journal.pone.0085565
Novel method to load multiple genes onto a mammalian artificial chromosome
Abstract
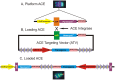
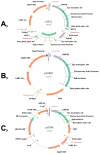
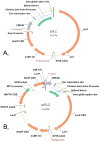
Mammalian artificial chromosomes are natural chromosome-based vectors that may carry a vast amount of genetic material in terms of both size and number. They are reasonably stable and segregate well in both mitosis and meiosis. A platform artificial chromosome expression system (ACEs) was earlier described with multiple loading sites for a modified lambda-integrase enzyme. It has been shown that this ACEs is suitable for high-level industrial protein production and the treatment of a mouse model for a devastating human disorder, Krabbe's disease. ACEs-treated mutant mice carrying a therapeutic gene lived more than four times longer than untreated counterparts. This novel gene therapy method is called combined mammalian artificial chromosome-stem cell therapy. At present, this method suffers from the limitation that a new selection marker gene should be present for each therapeutic gene loaded onto the ACEs. Complex diseases require the cooperative action of several genes for treatment, but only a limited number of selection marker genes are available and there is also a risk of serious side-effects caused by the unwanted expression of these marker genes in mammalian cells, organs and organisms. We describe here a novel method to load multiple genes onto the ACEs by using only two selectable marker genes. These markers may be removed from the ACEs before therapeutic application. This novel technology could revolutionize gene therapeutic applications targeting the treatment of complex disorders and cancers. It could also speed up cell therapy by allowing researchers to engineer a chromosome with a predetermined set of genetic factors to differentiate adult stem cells, embryonic stem cells and induced pluripotent stem (iPS) cells into cell types of therapeutic value. It is also a suitable tool for the investigation of complex biochemical pathways in basic science by producing an ACEs with several genes from a signal transduction pathway of interest.
Conflict of interest statement
Figures



References
-
- Seymour LW, Fisher KD (2009) Preclinical Screening of Gene Therapy in Human Tissues. Human Gene Therapy 20: 291–292. - PubMed
-
- Ascenzioni F, Donini P, Lipps HJ (1997) Mammalian artificial chromosomes–vectors for somatic gene therapy. Cancer Lett 118: 135–142. - PubMed
-
- Basu J, Willard HF (2006) Human artificial chromosomes: potential applications and clinical considerations. Pediatr Clin North Am 53: 843–853. - PubMed
-
- Bridger JM (2004) Mammalian artificial chromosomes: modern day feats of engineering–Isambard Kingdom Brunel style. Cytogenet Genome Res 107: 5–8. - PubMed
-
- Cooke H (2001) Mammalian artificial chromosomes as vectors: progress and prospects. Cloning Stem Cells 3: 243–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

