Softenin, a novel protein that softens the connective tissue of sea cucumbers through inhibiting interaction between collagen fibrils
- PMID: 24454910
- PMCID: PMC3893245
- DOI: 10.1371/journal.pone.0085644
Softenin, a novel protein that softens the connective tissue of sea cucumbers through inhibiting interaction between collagen fibrils
Abstract
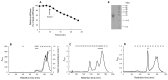
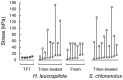
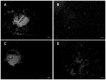
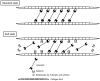
The dermis in the holothurian body wall is a typical catch connective tissue or mutable collagenous tissue that shows rapid changes in stiffness. Some chemical factors that change the stiffness of the tissue were found in previous studies, but the molecular mechanisms of the changes are not yet fully understood. Detection of factors that change the stiffness by working directly on the extracellular matrix was vital to clarify the mechanisms of the change. We isolated from the body wall of the sea cucumber Stichopus chloronotus a novel protein, softenin, that softened the body-wall dermis. The apparent molecular mass was 20 kDa. The N-terminal sequence of 17 amino acids had low homology to that of known proteins. We performed sequential chemical and physical dissections of the dermis and tested the effects of softenin on each dissection stage by dynamic mechanical tests. Softenin softened Triton-treated dermis whose cells had been disrupted by detergent. The Triton-treated dermis was subjected to repetitive freeze-and-thawing to make Triton-Freeze-Thaw (TFT) dermis that was softer than the Triton-treated dermis, implying that some force-bearing structure had been disrupted by this treatment. TFT dermis was stiffened by tensilin, a stiffening protein of sea cucumbers. Softenin softened the tensilin-stiffened TFT dermis while it had no effect on the TFT dermis without tensilin treatment. We isolated collagen from the dermis. When tensilin was applied to the suspending solution of collagen fibrils, they made a large compact aggregate that was dissolved by the application of softenin or by repetitive freeze-and-thawing. These results strongly suggested that softenin decreased dermal stiffness through inhibiting cross-bridge formation between collagen fibrils; the formation was augmented by tensilin and the bridges were broken by the freeze-thaw treatment. Softenin is thus the first softener of catch connective tissue shown to work on the cross-bridges between extracellular materials.
Conflict of interest statement
Figures




References
-
- Motokawa T (1984) Connective tissue catch in echinoderms. Biol Rev 59: 255–270.
-
- Motokawa T (1988) Catch connective tissue: a key character for echinoderms' success. In: Burke RD, Mladenov PV, Lambert P, Parsley RL, editors. Echinoderm Biology. Rotterdam, Brookfield: A. A. Balkema. pp. 39–54.
-
- Wilkie IC (2002) Is muscle involved in the mechanical adaptability of echinoderm mutable collagenous tissue? J Exp Biol 205: 159–165. - PubMed
-
- Trotter JA, Koob TJ (1989) Collagen and proteoglycan in a sea urchin ligament with mutable mechanical properties. Cell Tissue Res 258: 527–539. - PubMed
-
- Trotter JA, Thurmond FA, Koob TJ (1994) Molecular structure and functional morphology of echinoderm collagen fibrils. Cell Tissue Res 275: 451–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

