Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism
- PMID: 24454930
- PMCID: PMC3894207
- DOI: 10.1371/journal.pone.0085765
Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism
Abstract
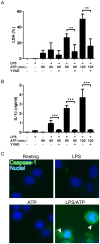
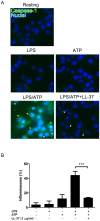
Pyroptosis is a caspase-1 dependent cell death, associated with proinflammatory cytokine production, and is considered to play a crucial role in sepsis. Pyroptosis is induced by the two distinct stimuli, microbial PAMPs (pathogen associated molecular patterns) and endogenous DAMPs (damage associated molecular patterns). Importantly, cathelicidin-related AMPs (antimicrobial peptides) have a role in innate immune defense. Notably, human cathelicidin LL-37 exhibits the protective effect on the septic animal models. Thus, in this study, to elucidate the mechanism for the protective action of LL-37 on sepsis, we utilized LPS (lipopolysaccharide) and ATP (adenosine triphosphate) as a PAMP and a DAMP, respectively, and examined the effect of LL-37 on the LPS/ATP-induced pyroptosis of macrophage-like J774 cells. The data indicated that the stimulation of J774 cells with LPS and ATP induces the features of pyroptosis, including the expression of IL-1β mRNA and protein, activation of caspase-1, inflammasome formation and cell death. Moreover, LL-37 inhibits the LPS/ATP-induced IL-1β expression, caspase-1 activation, inflammasome formation, as well as cell death. Notably, LL-37 suppressed the LPS binding to target cells and ATP-induced/P2X7-mediated caspase-1 activation. Together these observations suggest that LL-37 potently inhibits the LPS/ATP-induced pyroptosis by both neutralizing the action of LPS and inhibiting the response of P2X7 to ATP. Thus, the present finding may provide a novel insight into the modulation of sepsis utilizing LL-37 with a dual action on the LPS binding and P2X7 activation.
Conflict of interest statement
Figures



References
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, et al. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644–1655. - PubMed
-
- Angus DC (2011) The search for effective therapy for sepsis: back to the drawing board? Jama 306: 2614–2615. - PubMed
-
- O′Brien JM Jr, Ali NA, Aberegg SK, Abraham E (2007) Sepsis. Am J Med 120: 1012–1022. - PubMed
-
- Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, et al. (2011) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 38: 367–374. - PubMed
-
- Pinsky MR (2004) Dysregulation of the immune response in severe sepsis. Am J Med Sci 328: 220–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

