Protective effect of metalloporphyrins against cisplatin-induced kidney injury in mice
- PMID: 24454954
- PMCID: PMC3891880
- DOI: 10.1371/journal.pone.0086057
Protective effect of metalloporphyrins against cisplatin-induced kidney injury in mice
Abstract
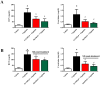
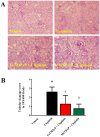
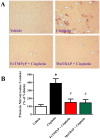
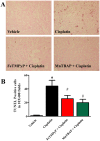
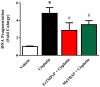
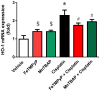
Oxidative and nitrative stress is a well-known phenomenon in cisplatin-induced nephrotoxicity. The purpose of this work is to study the role of two metalloporphyrins (FeTMPyP and MnTBAP), water soluble complexes, in cisplatin-induced renal damage and their ability to scavenge peroxynitrite. In cisplatin-induced nephropathy study in mice, renal nitrative stress was evident by the increase in protein nitration. Cisplatin-induced nephrotoxicity was also evident by the histological damage from the loss of the proximal tubular brush border, blebbing of apical membranes, tubular epithelial cell detachment from the basement membrane, or intra-luminal aggregation of cells and proteins and by the increase in blood urea nitrogen and serum creatinine. Cisplatin-induced apoptosis and cell death as shown by Caspase 3 assessments, TUNEL staining and DNA fragmentation Cisplatin-induced nitrative stress, apoptosis and nephrotoxicity were attenuated by both metalloporphyrins. Heme oxygenase (HO-1) also plays a critical role in metalloporphyrin-mediated protection of cisplatin-induced nephrotoxicity. It is evident that nitrative stress plays a critical role in cisplatin-induced nephrotoxicity in mice. Our data suggest that peroxynitrite is involved, at least in part, in cisplatin-induced nephrotoxicity and protein nitration and cisplatin-induced nephrotoxicity can be prevented with the use of metalloporphyrins.
Conflict of interest statement
Figures


Similar articles
-
5-Aminolevulinic acid protects against cisplatin-induced nephrotoxicity without compromising the anticancer efficiency of cisplatin in rats in vitro and in vivo.PLoS One. 2013 Dec 6;8(12):e80850. doi: 10.1371/journal.pone.0080850. eCollection 2013. PLoS One. 2013. PMID: 24324635 Free PMC article.
-
Effects of ginsenoside Rh2 on cisplatin-induced nephrotoxicity in renal tubular epithelial cells by inhibiting endoplasmic reticulum stress.J Biochem Mol Toxicol. 2024 Aug;38(8):e23768. doi: 10.1002/jbt.23768. J Biochem Mol Toxicol. 2024. PMID: 39015062
-
Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury.Toxicology. 2014 Oct 3;324:98-107. doi: 10.1016/j.tox.2014.07.004. Epub 2014 Jul 15. Toxicology. 2014. PMID: 25043994
-
Cisplatin Nephrotoxicity: Novel Insights Into Mechanisms and Preventative Strategies.Semin Nephrol. 2022 Nov;42(6):151341. doi: 10.1016/j.semnephrol.2023.151341. Epub 2023 May 12. Semin Nephrol. 2022. PMID: 37182407 Review.
-
Molecular mechanisms underlying cisplatin-induced nephrotoxicity and the potential ameliorative effects of essential oils: A comprehensive review.Tissue Cell. 2024 Jun;88:102377. doi: 10.1016/j.tice.2024.102377. Epub 2024 Apr 6. Tissue Cell. 2024. PMID: 38626527 Review.
Cited by
-
Heat treatment and protective potentials of luteolin-7-O-glucoside against cisplatin genotoxic and cytotoxic effects.Environ Sci Pollut Res Int. 2020 Apr;27(12):13417-13427. doi: 10.1007/s11356-020-07900-7. Epub 2020 Feb 5. Environ Sci Pollut Res Int. 2020. PMID: 32026362
-
Kidney-Targeted Redox Scavenger Therapy Prevents Cisplatin-Induced Acute Kidney Injury.Front Pharmacol. 2022 Jan 3;12:790913. doi: 10.3389/fphar.2021.790913. eCollection 2021. Front Pharmacol. 2022. PMID: 35046813 Free PMC article.
-
Mn porphyrin-based SOD mimic, MnTnHex-2-PyP(5+), and non-SOD mimic, MnTBAP(3-), suppressed rat spinal cord ischemia/reperfusion injury via NF-κB pathways.Free Radic Res. 2014 Dec;48(12):1426-42. doi: 10.3109/10715762.2014.960865. Epub 2014 Oct 10. Free Radic Res. 2014. PMID: 25185063 Free PMC article.
-
Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death.JCI Insight. 2021 Jun 8;6(11):e148496. doi: 10.1172/jci.insight.148496. JCI Insight. 2021. PMID: 33914707 Free PMC article.
-
A Flavonoid-Rich Extract of Sambucus nigra L. Reduced Lipid Peroxidation in a Rat Experimental Model of Gentamicin Nephrotoxicity.Materials (Basel). 2022 Jan 20;15(3):772. doi: 10.3390/ma15030772. Materials (Basel). 2022. PMID: 35160718 Free PMC article.
References
-
- Fram RJ (1992) Cisplatin and platinum analogues: recent advances. Curr Opin Oncol 4: 1073–1079. - PubMed
-
- Dillioglugil MO, Maral Kir H, Gulkac MD, Ozon Kanli A, Ozdogan HK, et al. (2005) Protective effects of increasing vitamin E and a doses on cisplatin-induced oxidative damage to kidney tissue in rats. Urol Int 75: 340–344. - PubMed
-
- Srivastava RC, Farookh A, Ahmad N, Misra M, Hasan SK, et al. (1996) Evidence for the involvement of nitric oxide in cisplatin-induced toxicity in rats. Biometals 9: 139–142. - PubMed
-
- Davis CA, Nick HS, Agarwal A (2001) Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol 12: 2683–2690. - PubMed
-
- Kadikoylu G, Bolaman Z, Demir S, Balkaya M, Akalin N, et al. (2004) The effects of desferrioxamine on cisplatin-induced lipid peroxidation and the activities of antioxidant enzymes in rat kidneys. Hum Exp Toxicol 23: 29–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

