Production of pigs expressing a transgene under the control of a tetracycline-inducible system
- PMID: 24454957
- PMCID: PMC3893280
- DOI: 10.1371/journal.pone.0086146
Production of pigs expressing a transgene under the control of a tetracycline-inducible system
Abstract
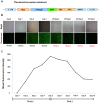
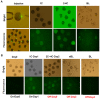
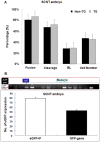
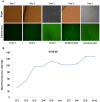
Pigs are anatomically and physiologically closer to humans than other laboratory animals. Transgenic (TG) pigs are widely used as models of human diseases. The aim of this study was to produce pigs expressing a tetracycline (Tet)-inducible transgene. The Tet-on system was first tested in infected donor cells. Porcine fetal fibroblasts were infected with a universal doxycycline-inducible vector containing the target gene enhanced green fluorescent protein (eGFP). At 1 day after treatment with 1 µg/ml doxycycline, the fluorescence intensity of these cells was increased. Somatic cell nuclear transfer (SCNT) was then performed using these donor cells. The Tet-on system was then tested in the generated porcine SCNT-TG embryos. Of 4,951 porcine SCNT-TG embryos generated, 850 were cultured in the presence of 1 µg/ml doxycycline in vitro. All of these embryos expressed eGFP and 15 embryos developed to blastocyst stage. The remaining 4,101 embryos were transferred to thirty three surrogate pigs from which thirty eight cloned TG piglets were obtained. PCR analysis showed that the transgene was inserted into the genome of each of these piglets. Two TG fibroblast cell lines were established from these TG piglets, and these cells were used as donor cells for re-cloning. The re-cloned SCNT embryos expressed the eGFP transgene under the control of doxycycline. These data show that the expression of transgenes in cloned TG pigs can be regulated by the Tet-on/off systems.
Conflict of interest statement
Figures


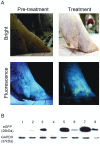
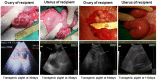
References
-
- Hemann M, Shen HG, Beach NM, Meng XJ, Halbur PG, et al. (2012) Expression of human CD46 has no effect on porcine circovirus type 2 infection and shedding in the experimental pig model. Vet Res Commun 36: 187–193. - PubMed
-
- Watanabe M, Kurome M, Matsunari H, Nakano K, Umeyema K, et al. (2012) The creation of transgenic pigs expressing human proteins using BAC-derived, full-length genes and intracytoplasmic sperm injection-mediated gene transfer. Transgenic Res 21: 605–618. - PubMed
-
- Giraldo AM, Ball S, Bondioli KR (2012) Production of transgenic and knockout pigs by somatic cell nuclear transfer. Methods Mol Biol 885: 105–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

