RHPS4 G-quadruplex ligand induces anti-proliferative effects in brain tumor cells
- PMID: 24454961
- PMCID: PMC3893285
- DOI: 10.1371/journal.pone.0086187
RHPS4 G-quadruplex ligand induces anti-proliferative effects in brain tumor cells
Abstract
Background: Telomeric 3' overhangs can fold into a four-stranded DNA structure termed G-quadruplex (G4), a formation which inhibits telomerase. As telomerase activation is crucial for telomere maintenance in most cancer cells, several classes of G4 ligands have been designed to directly disrupt telomeric structure.
Methods: We exposed brain tumor cells to the G4 ligand 3,11-difluoro-6,8,13-trimethyl-8H-quino[4,3,2-kl]acridinium methosulfate (RHPS4) and investigated proliferation, cell cycle dynamics, telomere length, telomerase activity and activated c-Myc levels.
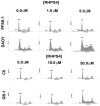
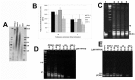
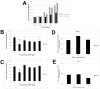
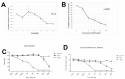
Results: Although all cell lines tested were sensitive to RHPS4, PFSK-1 central nervous system primitive neuroectodermal cells, DAOY medulloblastoma cells and U87 glioblastoma cells exhibited up to 30-fold increased sensitivity compared to KNS42 glioblastoma, C6 glioma and Res196 ependymoma cells. An increased proportion of S-phase cells were observed in medulloblastoma and high grade glioma cells whilst CNS PNET cells showed an increased proportion of G1-phase cells. RHPS4-induced phenotypes were concomitant with telomerase inhibition, manifested in a telomere length-independent manner and not associated with activated c-Myc levels. However, anti-proliferative effects were also observed in normal neural/endothelial cells in vitro and ex vivo.
Conclusion: This study warrants in vivo validation of RHPS4 and alternative G4 ligands as potential anti-cancer agents for brain tumors but highlights the consideration of dose-limiting tissue toxicities.
Conflict of interest statement
Figures


Similar articles
-
Pharmacodynamics of the G-quadruplex-stabilizing telomerase inhibitor 3,11-difluoro-6,8,13-trimethyl-8H-quino[4,3,2-kl]acridinium methosulfate (RHPS4) in vitro: activity in human tumor cells correlates with telomere length and can be enhanced, or antagonized, with cytotoxic agents.Mol Pharmacol. 2005 Dec;68(6):1551-8. doi: 10.1124/mol.105.013300. Epub 2005 Sep 8. Mol Pharmacol. 2005. PMID: 16150933
-
Biological activity of the G-quadruplex ligand RHPS4 (3,11-difluoro-6,8,13-trimethyl-8H-quino[4,3,2-kl]acridinium methosulfate) is associated with telomere capping alteration.Mol Pharmacol. 2004 Nov;66(5):1138-46. doi: 10.1124/mol.104.001537. Epub 2004 Aug 10. Mol Pharmacol. 2004. PMID: 15304549
-
Naphthalene diimide-derivatives G-quadruplex ligands induce cell proliferation inhibition, mild telomeric dysfunction and cell cycle perturbation in U251MG glioma cells.FEBS J. 2018 Oct;285(20):3769-3785. doi: 10.1111/febs.14628. Epub 2018 Sep 24. FEBS J. 2018. PMID: 30095224
-
G-quadruplexes as therapeutic targets.Biopolymers. 2000-2001;56(3):195-208. doi: 10.1002/1097-0282(2000)56:3<195::AID-BIP10009>3.0.CO;2-5. Biopolymers. 2000. PMID: 11745111 Review.
-
Targeting telomerase and telomeres to enhance ionizing radiation effects in in vitro and in vivo cancer models.Mutat Res Rev Mutat Res. 2017 Jul;773:204-219. doi: 10.1016/j.mrrev.2017.02.004. Epub 2017 Feb 24. Mutat Res Rev Mutat Res. 2017. PMID: 28927529 Review.
Cited by
-
Intragenic G-quadruplex structure formed in the human CD133 and its biological and translational relevance.Nucleic Acids Res. 2016 Feb 29;44(4):1579-90. doi: 10.1093/nar/gkv1122. Epub 2015 Oct 27. Nucleic Acids Res. 2016. PMID: 26511095 Free PMC article.
-
Possible molecular mechanisms underlying the development of atherosclerosis in cancer survivors.Front Cardiovasc Med. 2023 Jun 2;10:1186679. doi: 10.3389/fcvm.2023.1186679. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37332576 Free PMC article. Review.
-
Aging, Cancer, and Inflammation: The Telomerase Connection.Int J Mol Sci. 2024 Aug 5;25(15):8542. doi: 10.3390/ijms25158542. Int J Mol Sci. 2024. PMID: 39126110 Free PMC article. Review.
-
Exploring the Interaction of G-quadruplex Binders with a (3 + 1) Hybrid G-quadruplex Forming Sequence within the PARP1 Gene Promoter Region.Molecules. 2022 Jul 26;27(15):4792. doi: 10.3390/molecules27154792. Molecules. 2022. PMID: 35897968 Free PMC article.
-
General commentary: GQIcombi application to subdue glioma via differentiation therapy.Front Oncol. 2024 Dec 12;14:1466102. doi: 10.3389/fonc.2024.1466102. eCollection 2024. Front Oncol. 2024. PMID: 39726709 Free PMC article. No abstract available.
References
-
- Blackburn EH (1991) Structure and function of telomeres. Nature 350: 569–573. - PubMed
-
- Blasco MA (2004) Telomere epigenetics: a higher-order control of telomere length in mammalian cells. Carcinogenesis 25: 1083–1087. - PubMed
-
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70. - PubMed
-
- Harley CB, Kim NW, Prowse KR, Weinrich SL, Hirsch KS, et al. (1994) Telomerase, cell immortality and cancer. Cold Spring Harbor Symposia on Quantitative Biology 59: 307–315. - PubMed
-
- Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, et al. (2005) In vivo inhibition of lung cancer by GRN163L: A novel human telomerase inhibitor. Cancer Research 65: 7866–7873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

