Sequence comparison for non-enhanced MRA of the lower extremity arteries at 7 Tesla
- PMID: 24454963
- PMCID: PMC3894206
- DOI: 10.1371/journal.pone.0086274
Sequence comparison for non-enhanced MRA of the lower extremity arteries at 7 Tesla
Abstract
In this study three sequences for non-contrast-enhanced MRA of the lower extremity arteries at 7T were compared. Cardiac triggering was used with the aim to reduce signal variations in the arteries. Two fast single-shot 2D sequences, a modified Ultrafast Spoiled Gradient Echo (UGRE) sequence and a variant of the Quiescent-Interval Single-Shot (QISS) sequence were triggered via phonocardiogram and compared in volunteer examinations to a non-triggered 2D gradient echo (GRE) sequence. For image acquisition, a 16-channel transmit/receive coil and a manually positionable AngioSURF table were used. To tackle B1 inhomogeneities at 7T, Time-Interleaved Acquisition of Modes (TIAMO) was integrated in GRE and UGRE. To compare the three sequences quantitatively, a vessel-to-background ratio (VBR) was measured in all volunteers and stations. In conclusion, cardiac triggering was able to suppress flow artifacts satisfactorily. The modified UGRE showed only moderate image artifacts. Averaged over all volunteers and stations, GRE reached a VBR of 4.18±0.05, UGRE 5.20±0.06, and QISS 2.72±0.03. Using cardiac triggering and TIAMO imaging technique was essential to perform non-enhanced MRA of the lower extremities vessels at 7T. The modified UGRE performed best, as observed artifacts were only moderate and the highest average VBR was reached.
Conflict of interest statement
Figures


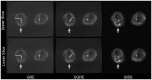
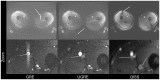

References
-
- Gutzeit A, Sutter R, Froehlich JM, Roos JE, Sautter T, et al. (2011) ECG-triggered non-contrast-enhanced MR angiography (TRANCE) versus digital subtraction angiography (DSA) in patients with peripheral arterial occlusive disease of the lower extremities. Eur Radiol 21: 1979–1987. - PubMed
-
- Prince MR, Zhang HL, Roditi GH, Leiner T, Kucharczyk W (2009) Risk factors for NSF: a literature review. J Magn Reson Imaging 30: 1298–1308. - PubMed
-
- Lanzman RS, Blondin D, Schmitt P, Orzechowski D, Godehardt E, et al. (2010) Non-Enhanced 3D MR Angiography of the Lower Extremity using ECG-Gated TSE Imaging with Non-Selective Refocusing Pulses - Initial Experience. Rofo 182: 861–867. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

