Safety and efficacy of single dose versus multiple doses of AmBisome for treatment of visceral leishmaniasis in eastern Africa: a randomised trial
- PMID: 24454970
- PMCID: PMC3894173
- DOI: 10.1371/journal.pntd.0002613
Safety and efficacy of single dose versus multiple doses of AmBisome for treatment of visceral leishmaniasis in eastern Africa: a randomised trial
Abstract
Background: Anti-leishmanial drug regimens that include a single dose AmBisome could be suitable for eastern African patients with symptomatic visceral leishmaniasis (VL) but the appropriate single dose is unknown.
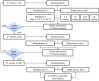
Methodology: A multi-centre, open-label, non-inferiority, randomized controlled trial with an adaptive design, was conducted to compare the efficacy and safety of a single dose and multiple doses of AmBisome for the treatment of VL in eastern Africa. The primary efficacy endpoint was definitive cure (DC) at 6 months. Symptomatic patients with parasitologically-confirmed, non-severe VL, received a single dose of AmBisome 7.5 mg/kg body weight or multiple doses, 7 times 3 mg/kg on days 1-5, 14, and 21. If interim analyses, evaluated 30 days after the start of treatment following 40 or 80 patients, showed the single dose gave significantly poorer parasite clearance than multiple doses at the 5% significance level, the single dose was increased by 2·5 mg/kg. In a sub-set of patients, parasite clearance was measured by quantitative reverse transcriptase (qRT) PCR.
Principal findings: The trial was terminated after the third interim analysis because of low efficacy of both regimens. Based on the intention-to-treat population, DC was 85% (95%CI 73-93%), 40% (95%CI 19-64%), and 58% (95%CI 41-73%) in patients treated with multiple doses (n = 63), and single doses of 7·5 (n = 21) or 10 mg/kg (n = 40), respectively. qRT-PCR suggested superior parasite clearance with multiple doses as early as day 3. Safety data accorded with the drug label.
Conclusions: The tested AmBisome regimens would not be suitable for VL treatment across eastern Africa. An optimal single dose regimen was not identified.
Trials registration: www.clinicaltrials.govNCT00832208.
Conflict of interest statement
The authors have declared that no competing interests exist. Gilead Sciences, USA, provided the investigational product, AmBisome®. Gilead Sciences and the sponsors, DNDi, have professional collaborations. This collaboration does not alter our adherence to all PLOS NTDs policies on sharing data and materials.
Figures

References
-
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, et al. (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7 (5) e35671 doi:10.1371/journal. pone.0035671 - DOI - PMC - PubMed
-
- WHO (2007) Fifth Consultative Meeting on Leishmania/HIV Coinfection, Addis Ababa, Ethiopia, 20–22 March 2007. World Health Organisation, WHO/CDS/NTD/IDM/20075.
-
- Zijlstra EE, El-Hassan AM (2001) Leishmaniasis in Sudan. Visceral leishmaniasis. Trans R Soc Trop Med Hyg 95, Suppl 1: S27–S58. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

