Goniomitine: an overview on the chemistry of this indole alkaloid
- PMID: 24455296
- PMCID: PMC3884615
- DOI: 10.1155/2013/292396
Goniomitine: an overview on the chemistry of this indole alkaloid
Abstract
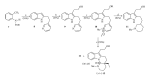
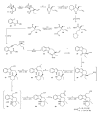
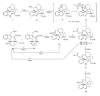
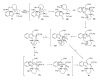
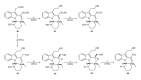
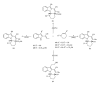
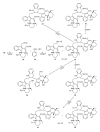
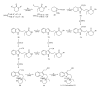
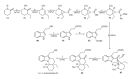
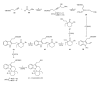
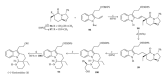
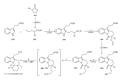
This paper reports an overview on the chemistry of the indole alkaloid goniomitine focusing, mainly, on the methods of synthesis related to this natural product and analogs.
Figures




Similar articles
-
Total Synthesis of (±)-Goniomitine via Radical Translocation.Org Lett. 2015 Sep 18;17(18):4558-60. doi: 10.1021/acs.orglett.5b02277. Epub 2015 Sep 8. Org Lett. 2015. PMID: 26348647
-
Scalable Enantioselective Total Synthesis of (-)-Goniomitine.Angew Chem Int Ed Engl. 2019 Jan 21;58(4):1174-1177. doi: 10.1002/anie.201812822. Epub 2018 Dec 20. Angew Chem Int Ed Engl. 2019. PMID: 30520199
-
Total syntheses of (-)- and (+)-goniomitine.Org Lett. 2011 Apr 1;13(7):1796-9. doi: 10.1021/ol200320z. Epub 2011 Mar 4. Org Lett. 2011. PMID: 21375315
-
Marine Natural Product Bis-indole Alkaloid Caulerpin: Chemistry and Biology.Mini Rev Med Chem. 2019;19(9):751-761. doi: 10.2174/1389557517666170927154231. Mini Rev Med Chem. 2019. PMID: 28971770 Review.
-
[Development of new synthetic methods and their application to synthesis of useful compounds].Yakugaku Zasshi. 2003 Dec;123(12):1007-21. doi: 10.1248/yakushi.123.1007. Yakugaku Zasshi. 2003. PMID: 14689864 Review. Japanese.
References
-
- Saxton JE. Recent progress in the chemistry of the monoterpenoid indole alkaloids. Natural Product Reports. 1997;14(6):559–590. - PubMed
-
- El-Sayed M, Verpoorte R. Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochemistry Reviews. 2007;6(2-3):277–305.
-
- Frederich M, Tits M, Angenot L. Potential antimalarial activity of indole alkaloids. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(1):11–19. - PubMed
-
- Ziegler J, Facchini PJ. Alkaloid biosynthesis: metabolism and trafficking. Annual Review of Plant Biology. 2008;59:735–769. - PubMed
-
- Hájícek J. Recent developments in syntheses of the post-secodine indole alkaloids. Part III: rearranged alkaloid types. Collection of Czechoslovak Chemical Communications. 2011;76(12):2023–2083.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

