Amodiaquine-Artesunate versus Artemether-Lumefantrine against Uncomplicated Malaria in Children Less Than 14 Years in Ngaoundere, North Cameroon: Efficacy, Safety, and Baseline Drug Resistant Mutations in pfcrt, pfmdr1, and pfdhfr Genes
- PMID: 24455414
- PMCID: PMC3876914
- DOI: 10.1155/2013/234683
Amodiaquine-Artesunate versus Artemether-Lumefantrine against Uncomplicated Malaria in Children Less Than 14 Years in Ngaoundere, North Cameroon: Efficacy, Safety, and Baseline Drug Resistant Mutations in pfcrt, pfmdr1, and pfdhfr Genes
Retraction in
-
Retracted: Amodiaquine-Artesunate versus Artemether-Lumefantrine against Uncomplicated Malaria in Children Less Than 14 Years in Ngaoundere, North Cameroon: Efficacy, Safety, and Baseline Drug Resistant Mutations in pfcrt, pfmdr1, and pfdhfr Genes.Malar Res Treat. 2019 Jun 20;2019:4274315. doi: 10.1155/2019/4274315. eCollection 2019. Malar Res Treat. 2019. PMID: 31321025 Free PMC article.
Abstract
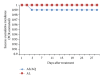
Background. In Cameroon, both Artesunate-amodiaquine (AS/AQ) and artemether-lumefantrine (AL) are used as first-line treatment against uncomplicated malaria in line with the WHO recommendations. We compared the efficacy and safety of both therapeutic combinations and determined the prevalence of drug resistance conferring mutations in three parasite genes. Methods. One hundred and fifty acute malaria patients between six months and 14 years of age were randomized to receive standard doses of either AS/AQ (73) or AL (77) and followedup for 28 days. Outcome of treatment was according to the standard WHO classification. DNA samples from pretreatment parasite isolates were used to determine the prevalence of resistant mutations in the pfcrt, pfmdr1, and dhfr genes. Results. Both drug combinations induced rapid clearance of parasites and malaria symptoms. PCR-corrected cure rates were 100% and 96.4% for AL. The combinations were well tolerated. Major haplotypes included CVIET (71%), CVMNT (25%) for the pfcrt; SND (100%) for the pfmdr1; IRN (79, 8%), NCS (8.8%), and mixed haplotype (11, 8%) for the dhfr. Conclusion. Both AS/AQ and AL were highly effective and well tolerated for the treatment of uncomplicated falciparum malaria in Ngaoundere, Cameroon. High prevalence of mutant pfcrt alleles confirms earlier observations. Long-term monitoring of safety and efficacy and molecular markers is highly solicited.
Figures



References
-
- WHO. Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization; 2006.
-
- Attaran A, Barnes KI, Curtis C, et al. WHO, the global fund, and medical malpractice in malaria treatment. The Lancet. 2004;363(9404):237–240. - PubMed
-
- Bloland PB, Ettling M. Making malaria-treatment policy in the face of drug resistance. Annals of Tropical Medicine and Parasitology. 1999;93(1):5–23. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

