Electrochemical analysis of antichemotherapeutic drug zanosar in pharmaceutical and biological samples by differential pulse polarography
- PMID: 24455423
- PMCID: PMC3886556
- DOI: 10.1155/2013/420761
Electrochemical analysis of antichemotherapeutic drug zanosar in pharmaceutical and biological samples by differential pulse polarography
Abstract
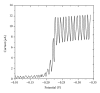
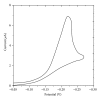
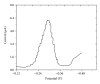
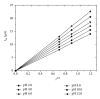
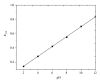
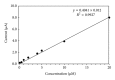
The electrochemical reduction of zanosar was investigated systematically by direct current polarography, cyclic voltammetry, and differential pulse polarography (DPP). A simple DPP technique was proposed for the direct quantitative determination of anticancer drug zanosar in pharmaceutical formulation and spiked human urine samples for the first time. The reduction potential was -0.28 V versus Ag/AgCl with a hanging mercury drop electrode in Britton-Robinson buffer as supporting electrolyte. The dependence of the intensities of currents and potentials on pH, concentration, scan rate, deposition time, and nature of the supporting electrolyte was investigated. The calibration curve was found to be linear with the following equation: y = 0.4041x + 0.012, with a correlation coefficient of 0.992 (R (2)) over a concentration range from 1.0 × 10(-7) M to 1.0 × 10(-3) M. In the present investigation, the achieved limit of detection (LOD) and limit of quantization (LQD) were 7.42 × 10(-8) M and 2.47 × 10(-8) M; respectively. Excipients did not interfere with the determination of zanosar in pharmaceutical formulation and spiked urine samples. Precision and accuracy of the developed method were checked by recovery studies in pharmaceutical formulation and spiked human urine samples.
Figures
Similar articles
-
Electrochemical study of spiramycin and its determination in pharmaceutical preparation.Drug Test Anal. 2010 Aug;2(8):392-6. doi: 10.1002/dta.137. Drug Test Anal. 2010. PMID: 20836146
-
Adsorptive stripping voltammetric assay of phenazopyridine hydrochloride in biological fluids and pharmaceutical preparations.Talanta. 1999 Aug 23;50(1):133-40. doi: 10.1016/s0039-9140(99)00113-7. Talanta. 1999. PMID: 18967703
-
Stripping voltammetric and polarographic techniques for the determination of anti-fungal ketoconazole on the mercury electrode.J Pharm Biomed Anal. 2003 Nov 24;33(4):589-96. doi: 10.1016/s0731-7085(03)00247-4. J Pharm Biomed Anal. 2003. PMID: 14623584
-
Electrochemical Behaviour of Tinidazole at 1,4-Benzoquinone Modified Carbon Paste Electrode and Its Direct Determination in Pharmaceutical Tablets and Urine by Differential Pulse Voltammetry.J Anal Methods Chem. 2017;2017:8518707. doi: 10.1155/2017/8518707. Epub 2017 Nov 7. J Anal Methods Chem. 2017. PMID: 29250454 Free PMC article.
-
Assay of the anti-psychotic drug haloperidol in bulk form, pharmaceutical formulation and biological fluids using square-wave adsorptive stripping voltammetry at a mercury electrode.J Pharm Biomed Anal. 2005 Jul 1;38(3):543-50. doi: 10.1016/j.jpba.2005.01.017. Epub 2005 Feb 26. J Pharm Biomed Anal. 2005. PMID: 15925258
References
-
- Vavra JJ, Deboer C, Dietz A, Hanka LJ, Sokolski WT. Streptozotocin, a new antibacterial antibiotic. Antibiotics Annual. 1959;7:230–235. - PubMed
-
- Mansford KR, Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. The Lancet. 1968;1(7544):670–671. - PubMed
-
- Rerup CC. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacological Reviews. 1970;22(4):485–518. - PubMed
-
- Murray-Lyon IM, Eddleston AL, Williams R, et al. Treatment of multiple-hormone-producing malignant islet-cell tumour with streptozotocin. The Lancet. 1968;2(7574):895–898. - PubMed
-
- Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist’s perspective. Surgical Clinics of North America. 2001;81(3):527–542. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

