Exploring the retention properties of CaF2 nanoparticles as possible additives for dental care application with tapping-mode atomic force microscope in liquid
- PMID: 24455460
- PMCID: PMC3896269
- DOI: 10.3762/bjnano.5.4
Exploring the retention properties of CaF2 nanoparticles as possible additives for dental care application with tapping-mode atomic force microscope in liquid
Abstract
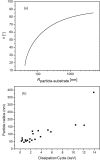
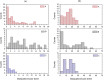
Amplitude-modulation atomic force microscopy (AM-AFM) is used to determine the retention properties of CaF2 nanoparticles adsorbed on mica and on tooth enamel in liquid. From the phase-lag of the forced cantilever oscillation the local energy dissipation at the detachment point of the nanoparticle was determined. This enabled us to compare different as-synthesized CaF2 nanoparticles that vary in shape, size and surface structure. CaF2 nanoparticles are candidates for additives in dental care products as they could serve as fluorine-releasing containers preventing caries during a cariogenic acid attack on the teeth. We show that the adherence of the nanoparticles is increased on the enamel substrate compared to mica, independently of the substrate roughness, morphology and size of the particles.
Keywords: AM-AFM in liquid; nanodentistry; nanoparticles.
Figures


Similar articles
-
Silica nanoparticles to polish tooth surfaces for caries prevention.J Dent Res. 2008 Oct;87(10):980-3. doi: 10.1177/154405910808701007. J Dent Res. 2008. PMID: 18809755
-
Preparation and optimization of calcium fluoride particles for dental applications.J Mater Sci Mater Med. 2014 Jul;25(7):1671-7. doi: 10.1007/s10856-014-5200-x. Epub 2014 Mar 30. J Mater Sci Mater Med. 2014. PMID: 24682907
-
Tapping mode imaging and measurements with an inverted atomic force microscope.Langmuir. 2006 Jul 18;22(15):6701-6. doi: 10.1021/la060002i. Langmuir. 2006. PMID: 16831016
-
[The cariostatic mechanisms of action of fluorides. A review].Schweiz Monatsschr Zahnmed. 1995;105(3):311-7. Schweiz Monatsschr Zahnmed. 1995. PMID: 7716463 Review. German.
-
Nature and role of loosely bound fluoride in dental caries.J Dent Res. 1990 Feb;69 Spec No:601-5; discussion 634-6. doi: 10.1177/00220345900690S118. J Dent Res. 1990. PMID: 2179320 Review.
Cited by
-
Periapical healing in endodontic retreatment: A comparative study of nano-hydroxyapatite with and without PRF.Bioinformation. 2025 May 31;21(5):1195-1200. doi: 10.6026/973206300211195. eCollection 2025. Bioinformation. 2025. PMID: 40822788 Free PMC article.
References
-
- Tamayo J, García R. Langmuir. 1996;12:4430–4435. doi: 10.1021/la960189l. - DOI
-
- Magonov S N, Elings V, Whangbo M-H. Surf Sci. 1997;375:L385–L391. doi: 10.1016/S0039-6028(96)01591-9. - DOI
-
- Cleveland J P, Anczykowski B, Schmid A E, Elings V B. Appl Phys Lett. 1998;72:2613. doi: 10.1063/1.121434. - DOI
-
- Anczykowski B, Gotsmann B, Fuchs H, Cleveland J P, Elings V B. Appl Surf Sci. 1999;140:376–382. doi: 10.1016/S0169-4332(98)00558-3. - DOI
-
- Tamayo J, García R. Appl Phys Lett. 1998;73:2926. doi: 10.1063/1.122632. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
