Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions
- PMID: 24455476
- PMCID: PMC3895620
- DOI: 10.1016/j.redox.2013.12.011
Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions
Abstract
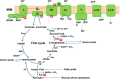
Mitochondria have a myriad of essential functions including metabolism and apoptosis. These chief functions are reliant on electron transfer reactions and the production of ATP and reactive oxygen species (ROS). The production of ATP and ROS are intimately linked to the electron transport chain (ETC). Electrons from nutrients are passed through the ETC via a series of acceptor and donor molecules to the terminal electron acceptor molecular oxygen (O2) which ultimately drives the synthesis of ATP. Electron transfer through the respiratory chain and nutrient oxidation also produces ROS. At high enough concentrations ROS can activate mitochondrial apoptotic machinery which ultimately leads to cell death. However, if maintained at low enough concentrations ROS can serve as important signaling molecules. Various regulatory mechanisms converge upon mitochondria to modulate ATP synthesis and ROS production. Given that mitochondrial function depends on redox reactions, it is important to consider how redox signals modulate mitochondrial processes. Here, we provide the first comprehensive review on how redox signals mediated through cysteine oxidation, namely S-oxidation (sulfenylation, sulfinylation), S-glutathionylation, and S-nitrosylation, regulate key mitochondrial functions including nutrient oxidation, oxidative phosphorylation, ROS production, mitochondrial permeability transition (MPT), apoptosis, and mitochondrial fission and fusion. We also consider the chemistry behind these reactions and how they are modulated in mitochondria. In addition, we also discuss emerging knowledge on disorders and disease states that are associated with deregulated redox signaling in mitochondria and how mitochondria-targeted medicines can be utilized to restore mitochondrial redox signaling.
Keywords: Mitochondria; Redox; S-glutathionylation; S-nitrosylation; S-oxidation.
Figures



References
-
- Brown G.C., Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 2012;12:1–4. - PubMed
-
- Fernie A.R., Carrari F., Sweetlove L.J. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004;7:254–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

