Engineered hypopharynx from coculture of epithelial cells and fibroblasts using poly(ester urethane) as substratum
- PMID: 24455669
- PMCID: PMC3881389
- DOI: 10.1155/2013/138504
Engineered hypopharynx from coculture of epithelial cells and fibroblasts using poly(ester urethane) as substratum
Abstract
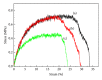
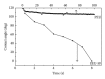
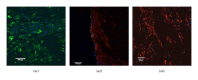
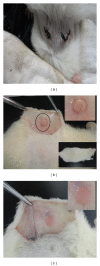
Porous polymeric scaffolds have been much investigated and applied in the field of tissue engineering research. Poly(ester urethane) (PEU) scaffolds, comprising pores of 1-20 μ m in diameter on one surface and ≥ 200 μ m on the opposite surface and in bulk, were fabricated using phase separation method for hypopharyngeal tissue engineering. The scaffolds were grafted with silk fibroin (SF) generated from natural silkworm cocoon to enhance the scaffold's hydrophilicity and further improve cytocompatibility to both primary epithelial cells (ECs) and fibroblasts of human hypopharynx tissue. Coculture of ECs and fibroblasts was conducted on the SF-grafted PEU scaffold (PEU-SF) to evaluate its in vitro cytocompatibility. After co-culture for 14 days, ECs were lined on the scaffold surface while fibroblasts were distributed in scaffold bulk. The results of in vivo investigation showed that PEU porous scaffold possessed good biocompatibility after it was grafted by silk fibroin. SF grafting improved the cell/tissue infiltration into scaffold bulk. Thus, PEU-SF porous scaffold is expected to be a good candidate to support the hypopharynx regeneration.
Figures






Similar articles
-
Surface modification of polyurethane towards promoting the ex vivo cytocompatibility and in vivo biocompatibility for hypopharyngeal tissue engineering.J Biomater Appl. 2013 Nov;28(4):607-16. doi: 10.1177/0885328212468184. Epub 2012 Dec 14. J Biomater Appl. 2013. PMID: 23241963
-
In vitro construction and in vivo regeneration of esophageal bilamellar muscle tissue.J Biomater Appl. 2016 Apr;30(9):1373-84. doi: 10.1177/0885328215627585. Epub 2016 Jan 27. J Biomater Appl. 2016. PMID: 26823400
-
Fabrication and evaluation of poly(epsilon-caprolactone)/silk fibroin blend nanofibrous scaffold.Biopolymers. 2012 May;97(5):265-75. doi: 10.1002/bip.22016. Epub 2011 Dec 14. Biopolymers. 2012. PMID: 22169927
-
Nanohydroxyapatite/poly(ester urethane) scaffold for bone tissue engineering.Acta Biomater. 2009 Nov;5(9):3316-27. doi: 10.1016/j.actbio.2009.05.001. Epub 2009 May 13. Acta Biomater. 2009. PMID: 19442765
-
Electrospun silk fibroin/poly(lactide-co-ε-caprolactone) nanofibrous scaffolds for bone regeneration.Int J Nanomedicine. 2016 Apr 11;11:1483-500. doi: 10.2147/IJN.S97445. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27114708 Free PMC article.
Cited by
-
Decellularized small intestine submucosa/polylactic-co-glycolic acid composite scaffold for potential application in hypopharyngeal and cervical esophageal tissue repair.Regen Biomater. 2021 Mar 13;8(2):rbaa061. doi: 10.1093/rb/rbaa061. eCollection 2021 Mar. Regen Biomater. 2021. PMID: 33738114 Free PMC article.
-
Intraperitoneal co-administration of low dose urethane with xylazine and ketamine for extended duration of surgical anesthesia in rats.Lab Anim Res. 2015 Dec;31(4):174-9. doi: 10.5625/lar.2015.31.4.174. Epub 2015 Dec 22. Lab Anim Res. 2015. PMID: 26755920 Free PMC article.
References
-
- Patel D, Kuriakose M, Iyer S. Reconstruction of the laryngopharynx. Indian Journal of Plastic Surgery. 2007;40(12):S44–S51.
-
- Mihara M, Iida T, Hara H, et al. Reconstruction of the larynx and aryepiglottic fold using a free radial forearm tendocutaneous flap after partial laryngopharyngectomy: a case report. Microsurgery. 2012;32(1):50–54. - PubMed
-
- Disa JJ, Pusic AL, Hidalgo DA, Cordeiro PG. Microvascular reconstruction of the hypopharynx: defect classification, treatment algorithm, and functional outcome based on 165 consecutive cases. Plastic and Reconstructive Surgery. 2003;111(2):652–660. - PubMed
-
- Yu P, Robb GL. Pharyngoesophageal reconstruction with the anterolateral thigh flap: a clinical and functional outcomes study. Plastic and Reconstructive Surgery. 2005;116(7):1845–1855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

