Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses
- PMID: 24456586
- PMCID: PMC4016573
- DOI: 10.1186/1756-8722-7-11
Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses
Abstract
Background: Romidepsin is a structurally unique, potent, bicyclic class 1 selective histone deacetylase inhibitor approved by the US Food and Drug Administration for the treatment of patients with cutaneous T-cell lymphoma who have received ≥ 1 prior systemic therapy and patients with peripheral T-cell lymphoma (PTCL) who have received ≥ 1 prior therapy. Approval for PTCL was based on results (n = 130; median follow-up, 13.4 months) from the pivotal study of romidepsin for the treatment of relapsed/refractory PTCL. The objective is to present updated data (median follow-up, 22.3 months) and to characterize patients who achieved long-term responses (≥ 12 months) to romidepsin.
Methods: Patients with PTCL who relapsed from or were refractory to ≥ 1 prior systemic therapy received romidepsin 14 mg/m2 as a 4-hour intravenous infusion on days 1, 8, and 15 every 28 days for up to 6 cycles; patients with response or stable disease could continue romidepsin beyond 6 cycles. The primary endpoint was rate of confirmed/unconfirmed complete response (CR/CRu) determined by an Independent Review Committee. Secondary endpoints included objective response rate (ORR) and duration of response (DOR). For patients who achieved CR/CRu, baseline characteristics by DOR (≥ 12 vs < 12 months) were examined.
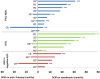
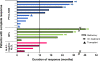
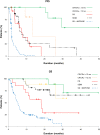
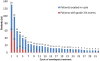
Results: The ORR to romidepsin was 25%, including 15% with CR/CRu. The median DOR for all responders was 28 months (range, < 1-48+) and was not reached for those who achieved CR/CRu. Patients with lack of response or transient response to prior therapy achieved durable responses with romidepsin. Of the 19 patients who achieved CR/CRu, 10 had long-term (≥ 12 months) responses; none of the baseline characteristics examined-including heavy pretreatment, response to prior therapy, or advanced disease-precluded long-term responses to romidepsin. With a median progression-free survival of 29 months, patients who achieved CR/CRu for ≥ 12 months had significantly longer survival vs those with CR/CRu for < 12 months or < CR/CRu. Extended treatment and longer follow-up did not affect the reported safety profile of romidepsin.
Conclusions: Treatment with romidepsin leads to highly durable responses in a subset of patients with relapsed/refractory PTCL, with responses ongoing as long as 48 months.
Trial registration: ClinicalTrials.gov NCT00426764.
Figures

References
-
- Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. - PubMed
-
- Ascani S, Zinzani PL, Gherlinzoni F, Sabattini E, Briskomatis A, de Vivo A, Piccioli M, Fraternali Orcioni G, Pieri F, Goldoni A. et al. Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the R.E.A.L. classification. Ann Oncol. 1997;8(6):583–592. doi: 10.1023/A:1008200307625. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

