Pharmacokinetics, hemodynamic and metabolic effects of epinephrine to prevent post-operative low cardiac output syndrome in children
- PMID: 24456639
- PMCID: PMC4056810
- DOI: 10.1186/cc13707
Pharmacokinetics, hemodynamic and metabolic effects of epinephrine to prevent post-operative low cardiac output syndrome in children
Abstract
Introduction: The response to exogenous epinephrine (Ep) is difficult to predict given the multitude of factors involved such as broad pharmacokinetic and pharmacodynamic between-subject variabilities, which may be more pronounced in children. We investigated the pharmacokinetics and pharmacodynamics of Ep, co-administered with milrinone, in children who underwent open heart surgical repair for congenital defects following cardiopulmonary bypass, including associated variability factors.
Methods: Thirty-nine children with a high risk of low cardiac output syndrome were prospectively enrolled. Ep pharmacokinetics, hemodynamic and metabolic effects were analyzed using the non-linear mixed effects modeling software MONOLIX. According to the final model, an Ep dosing simulation was suggested.
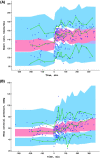
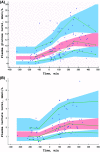
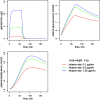
Results: Ep dosing infusions ranged from 0.01 to 0.23 μg.kg-1.min-1 in children whose weight ranged from 2.5 to 58 kg. A one-compartment open model with linear elimination adequately described the Ep concentration-time courses. Bodyweight (BW) was the main covariate influencing clearance (CL) and endogenous Ep production rate (q0) via an allometric relationship: CL(BWi) = θCL x (BWi)3/4 and q0(BWi) = θq0 x (BWi )3/4. The increase in heart rate (HR) and mean arterial pressure (MAP) as a function of Ep concentration were well described using an Emax model. The effect of age was significant on HR and MAP basal level parameters. Assuming that Ep stimulated the production rate of plasma glucose, the increases in plasma glucose and lactate levels were well described by turnover models without any significant effect of age, BW or exogenous glucose supply.
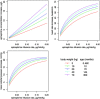
Conclusions: According to this population analysis, the developmental effects of BW and age explained a part of the pharmacokinetic and pharmacodynamics between-subject variabilities of Ep administration in critically ill children. This approach ultimately leads to a valuable Ep dosing simulation which should help clinicians to determine an appropriate a priori dosing regimen.
Figures


References
-
- Masse L, Antonacci M. Low cardiac output syndrome: identification and management. Crit Care Nurs Clin North Am. 2005;17:375–383. - PubMed
-
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. - PubMed
-
- Vogt W, Läer S. Prevention for pediatric low cardiac output syndrome results from the European survey EuLoCOS-Paed. Paediatr Anaesth. 2011;21:1176–1184. - PubMed
-
- Roth SJ, Adatia I, Pearson GD. Members of the Cardiology Group. Summary proceedings from the cardiology group on postoperative cardiac dysfunction. Pediatrics. 2006;117:S40–S46. - PubMed
-
- Fisher DG, Schwartz PH, Davis AL. Pharmacokinetics of exogenous epinephrine in critically ill children. Crit Care Med. 1993;21:111–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

