A subset of chicken statoacoustic ganglion neurites are repelled by Slit1 and Slit2
- PMID: 24456709
- PMCID: PMC3979322
- DOI: 10.1016/j.heares.2014.01.003
A subset of chicken statoacoustic ganglion neurites are repelled by Slit1 and Slit2
Abstract
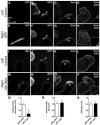
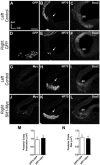
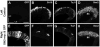
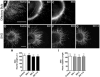
Mechanosensory hair cells in the chicken inner ear are innervated by bipolar afferent neurons of the statoacoustic ganglion (SAG). During development, individual SAG neurons project their peripheral process to only one of eight distinct sensory organs. These neuronal subtypes may respond differently to guidance cues as they explore the periphery in search of their target. Previous gene expression data suggested that Slit repellants might channel SAG neurites into the sensory primordia, based on the presence of robo transcripts in the neurons and the confinement of slit transcripts to the flanks of the prosensory domains. This led to the prediction that excess Slit proteins would impede the outgrowth of SAG neurites. As predicted, axonal projections to the primordium of the anterior crista were reduced 2-3 days after electroporation of either slit1 or slit2 expression plasmids into the anterior pole of the otocyst on embryonic day 3 (E3). The posterior crista afferents, which normally grow through and adjacent to slit expression domains as they are navigating towards the posterior pole of the otocyst, did not show Slit responsiveness when similarly challenged by ectopic delivery of slit to their targets. The sensitivity to ectopic Slits shown by the anterior crista afferents was more the exception than the rule: responsiveness to Slits was not observed when the entire E4 SAG was challenged with Slits for 40 h in vitro. The corona of neurites emanating from SAG explants was unaffected by the presence of purified human Slit1 and Slit2 in the culture medium. Reduced axon outgrowth from E8 olfactory bulbs cultured under similar conditions for 24 h confirmed bioactivity of purified human Slits on chicken neurons. In summary, differential sensitivity to Slit repellents may influence the directional outgrowth of otic axons toward either the anterior or posterior otocyst.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
-
- Adamska M, Herbrand H, Adamski M, Kruger M, Braun T, Bober E. FGFs control the patterning of the inner ear but are not able to induce the full ear program. Mech Dev. 2001;109:303–313. - PubMed
-
- Agarwala S, Sanders TA, Ragsdale CW. Sonic hedgehog control of size and shape in midbrain pattern formation. Science. 2001;291:2147–2150. - PubMed
-
- Bashaw GJ, Goodman CS. Chimeric axon guidance receptors: the cytoplasmic domains of slit and netrin receptors specify attraction versus repulsion. Cell. 1999;97:917–926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

