Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a gynecologic oncology group study
- PMID: 24456823
- PMCID: PMC4063288
- DOI: 10.1016/j.ygyno.2014.01.015
Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a gynecologic oncology group study
Abstract
Objectives: To determine the response, toxicities, and progression free survival of a regimen of temsirolimus with or without hormonal therapy in the treatment of advanced, or recurrent endometrial carcinoma.
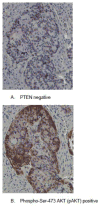
Background: Preclinical evidence suggested that blockade of the PI3K/AKT/mTOR pathway might overcome resistance to hormonal therapy.
Methods: We performed a randomized phase II trial of intravenous temsirolimus 25mg weekly versus the combination of weekly temsirolimus with a regimen of megestrol acetate 80 mg bid for three weeks alternating with tamoxifen 20mg bid for three weeks in women with recurrent or metastatic endometrial carcinoma.
Results: There were 71 eligible patients who received at least one dose of therapy with 21 of these treated on the combination arm which was closed early because of an excess of venous thrombosis, with 5 episodes of deep venous thrombosis (DVT) and 2 pulmonary emboli. There were three responses observed in that arm (14%). A total of 50 eligible patients were treated on the single agent arm with 3 episodes of DVT and 11 responses (22%). Response rates were similar in patients with prior chemotherapy (7 of 29; 24%) and those with no prior chemotherapy (4 of 21; 19%). Two of four patients with clear cell carcinoma responded.
Conclusions: Adding the combination of megestrol acetate and tamoxifen to temsirolimus therapy did not enhance activity and the combination was associated with an excess of venous thrombosis. Temsirolimus activity was preserved in patients with prior adjuvant chemotherapy.
Keywords: Endometrial cancer; Hormonal therapy; Megestrol acetate; Tamoxifen; Temsirolimus.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors wish to declare that there are no conflicts of interest.
Figures
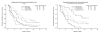

References
-
- Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–44. - PubMed
-
- Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:10–4. - PubMed
-
- Singh M, Zaino RJ, Filiaci VJ, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106:325–33. - PubMed
-
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–66. - PubMed
-
- Weigelt B, Banerjee S. Molecular targets and targeted therapeutics in endometrial cancer. Curr Opin Oncol. 2012;24:554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

