High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain
- PMID: 24457003
- PMCID: PMC3979109
- DOI: 10.1186/ar4449
High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain
Abstract
Introduction: Excessive mechanical loading of intervertebral discs (IVDs) is thought to alter matrix properties and influence disc cell metabolism, contributing to degenerative disc disease and development of discogenic pain. However, little is known about how mechanical strain induces these changes. This study investigated the cellular and molecular changes as well as which inflammatory receptors and cytokines were upregulated in human intervertebral disc cells exposed to high mechanical strain (HMS) at low frequency. The impact of these metabolic changes on neuronal differentiation was also explored to determine a role in the development of disc degeneration and discogenic pain.
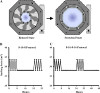
Methods: Isolated human annulus fibrosus (AF) and nucleus pulposus (NP) cells were exposed to HMS (20% cyclical stretch at 0.001 Hz) on high-extension silicone rubber dishes coupled to a mechanical stretching apparatus and compared to static control cultures. Gene expression of Toll-like receptors (TLRs), neuronal growth factor (NGF) and tumour necrosis factor α (TNFα) was assessed. Collected conditioned media were analysed for cytokine content and applied to rat pheocromocytoma PC12 cells for neuronal differentiation assessment.
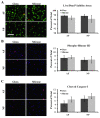
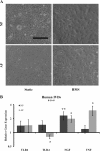
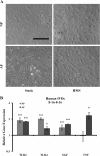
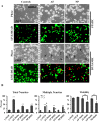
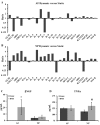
Results: HMS caused upregulation of TLR2, TLR4, NGF and TNFα gene expression in IVD cells. Medium from HMS cultures contained elevated levels of growth-related oncogene, interleukin 6 (IL-6), IL-8, IL-15, monocyte chemoattractant protein 1 (MCP-1), MCP-3, monokine induced by γ interferon, transforming growth factor β1, TNFα and NGF. Exposure of PC12 cells to HMS-conditioned media resulted in both increased neurite sprouting and cell death.
Conclusions: HMS culture of IVD cells in vitro drives cytokine and inflammatory responses associated with degenerative disc disease and low-back pain. This study provides evidence for a direct link between cellular strain, secretory factors, neoinnervation and potential degeneration and discogenic pain in vivo.
Figures
References
-
- Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;16:263–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

