Perilous journey: a tour of the ubiquitin-proteasome system
- PMID: 24457024
- PMCID: PMC4037451
- DOI: 10.1016/j.tcb.2013.12.003
Perilous journey: a tour of the ubiquitin-proteasome system
Abstract
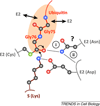
Eukaryotic cells are equipped to degrade proteins via the ubiquitin-proteasome system (UPS). Proteins become degraded upon their conjugation to chains of ubiquitin where they are then directed to the 26S proteasome, a macromolecular protease. The transfer of ubiquitin to proteins and their subsequent degradation are highly complex processes, and new research is beginning to uncover the molecular details of how ubiquitination and degradation take place in the cell. We review some of the new data providing insights into how these processes occur. Although distinct mechanisms are often observed, some common themes are emerging for how the UPS guides protein substrates through their final journey.
Keywords: E1-activating; E2-conjugating; E3-ligase; proteolysis; ubiquitin proteasome system.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

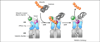
References
-
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009;78:399–434. - PubMed
-
- Tokunaga F, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat. Cell Biol. 2009;11:123–132. - PubMed
-
- Komander D, Rape M. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

