Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro
- PMID: 24457069
- PMCID: PMC3978713
- DOI: 10.1186/bcr3604
Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro
Abstract
Introduction: Upregulation of PI3K/Akt/mTOR signalling in endocrine-resistant breast cancer (BC) has identified mTOR as an attractive target alongside anti-hormones to control resistance. RAD001 (everolimus/Afinitor®), an allosteric mTOR inhibitor, is proving valuable in this setting; however, some patients are inherently refractory or relapse during treatment requiring alternative strategies. Here we evaluate the potential for novel dual mTORC1/2 mTOR kinase inhibitors, exemplified by AZD8055, by comparison with RAD001 in ER + endocrine resistant BC cells.
Methods: In vitro models of tamoxifen (TamR) or oestrogen deprivation resistance (MCF7-X) were treated with RAD001 or AZD8055 alone or combined with anti-hormone fulvestrant. Endpoints included growth, cell proliferation (Ki67), viability and migration, with PI3K/AKT/mTOR signalling impact monitored by Western blotting. Potential ER cross-talk was investigated by immunocytochemistry and RT-PCR.
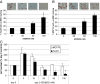
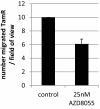
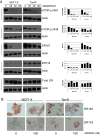
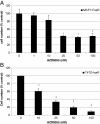
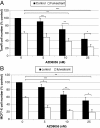
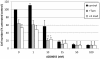
Results: RAD001 was a poor growth inhibitor of MCF7-derived TamR and MCF7-X cells (IC50 ≥1 μM), rapidly inhibiting mTORC1 but not mTORC2/AKT signalling. In contrast AZD8055, which rapidly inhibited both mTORC1 and mTORC2/AKT activity, was a highly effective (P <0.001) growth inhibitor of TamR (IC50 18 nM) and MCF7-X (IC50 24 nM), and of a further T47D-derived tamoxifen resistant model T47D-tamR (IC50 19 nM). AZD8055 significantly (P <0.05) inhibited resistant cell proliferation, increased cell death and reduced migration. Furthermore, dual treatment of TamR or MCF7-X cells with AZD8055 plus fulvestrant provided superior control of resistant growth versus either agent alone (P <0.05). Co-treating with AZD8055 alongside tamoxifen (P <0.01) or oestrogen deprivation (P <0.05) also effectively inhibited endocrine responsive MCF-7 cells. Although AZD8055 inhibited oestrogen receptor (ER) ser167 phosphorylation in TamR and MCF7-X, it had no effect on ER ser118 activity or expression of several ER-regulated genes, suggesting the mTOR kinase inhibitor impact was largely ER-independent. The capacity of AZD8055 for ER-independent activity was further evidenced by growth inhibition (IC5018 and 20 nM) of two acquired fulvestrant resistant models lacking ER.
Conclusions: This is the first report demonstrating dual mTORC1/2 mTOR kinase inhibitors have potential to control acquired endocrine resistant BC, even under conditions where everolimus fails. Such inhibitors may prove of particular benefit when used alongside anti-hormonal treatment as second-line therapy in endocrine resistant disease, and also potentially alongside anti-hormones during the earlier endocrine responsive phase to hinder development of resistance.
Figures



Similar articles
-
Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer.Breast Cancer Res. 2011 Mar 1;13(2):R21. doi: 10.1186/bcr2833. Breast Cancer Res. 2011. PMID: 21362200 Free PMC article.
-
Effectiveness and molecular interactions of the clinically active mTORC1 inhibitor everolimus in combination with tamoxifen or letrozole in vitro and in vivo.Breast Cancer Res. 2012 Oct 17;14(5):R132. doi: 10.1186/bcr3330. Breast Cancer Res. 2012. PMID: 23075476 Free PMC article.
-
AKT Antagonist AZD5363 Influences Estrogen Receptor Function in Endocrine-Resistant Breast Cancer and Synergizes with Fulvestrant (ICI182780) In Vivo.Mol Cancer Ther. 2015 Sep;14(9):2035-48. doi: 10.1158/1535-7163.MCT-15-0143. Epub 2015 Jun 26. Mol Cancer Ther. 2015. PMID: 26116361
-
Current development of the second generation of mTOR inhibitors as anticancer agents.Chin J Cancer. 2012 Jan;31(1):8-18. doi: 10.5732/cjc.011.10281. Epub 2011 Nov 4. Chin J Cancer. 2012. PMID: 22059905 Free PMC article. Review.
-
Aromatase inhibitors: combinations with fulvestrant or signal transduction inhibitors as a strategy to overcome endocrine resistance.J Steroid Biochem Mol Biol. 2005 May;95(1-5):173-81. doi: 10.1016/j.jsbmb.2005.04.004. J Steroid Biochem Mol Biol. 2005. PMID: 15996863 Review.
Cited by
-
Hyperactive mTOR and MNK1 phosphorylation of eIF4E confer tamoxifen resistance and estrogen independence through selective mRNA translation reprogramming.Genes Dev. 2017 Nov 15;31(22):2235-2249. doi: 10.1101/gad.305631.117. Epub 2017 Dec 21. Genes Dev. 2017. PMID: 29269484 Free PMC article.
-
High basal Wnt signaling is further induced by PI3K/mTor inhibition but sensitive to cSRC inhibition in mammary carcinoma cell lines with HER2/3 overexpression.BMC Cancer. 2015 Jul 25;15:545. doi: 10.1186/s12885-015-1544-y. BMC Cancer. 2015. PMID: 26205886 Free PMC article.
-
A low dose of AZD8055 enhances radiosensitivity of nasopharyngeal carcinoma cells by activating autophagy and apoptosis.Am J Cancer Res. 2019 Sep 1;9(9):1922-1937. eCollection 2019. Am J Cancer Res. 2019. PMID: 31598395 Free PMC article.
-
PDK1-mTOR signaling pathway inhibitors reduce cell proliferation in MK2206 resistant neuroblastoma cells.Cancer Cell Int. 2015 Sep 29;15:91. doi: 10.1186/s12935-015-0239-4. eCollection 2015. Cancer Cell Int. 2015. PMID: 26421002 Free PMC article.
-
Denoising perturbation signatures reveal an actionable AKT-signaling gene module underlying a poor clinical outcome in endocrine-treated ER+ breast cancer.Genome Biol. 2015 Apr 2;16(1):61. doi: 10.1186/s13059-015-0630-4. Genome Biol. 2015. PMID: 25886003 Free PMC article.
References
-
- Martin LA, Ghazoui Z, Weigel MT, Pancholi S, Dunbier A, Johnston S, Dowsett M. An in vitro model showing adaptation to long-term oestrogen deprivation highlights the clinical potential for targeting kinase pathways in combination with aromatase inhibition. Steroids. 2011;76:772–776. doi: 10.1016/j.steroids.2011.02.035. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

