3-Ketone-4,6-diene ceramide analogs exclusively induce apoptosis in chemo-resistant cancer cells
- PMID: 24457089
- PMCID: PMC3967587
- DOI: 10.1016/j.bmc.2013.12.065
3-Ketone-4,6-diene ceramide analogs exclusively induce apoptosis in chemo-resistant cancer cells
Abstract
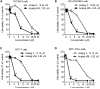
Multidrug-resistance is a major cause of cancer chemotherapy failure in clinical treatment. Evidence shows that multidrug-resistant cancer cells are as sensitive as corresponding regular cancer cells under the exposure to anticancer ceramide analogs. In this work we designed five new ceramide analogs with different backbones, in order to test the hypothesis that extending the conjugated system in ceramide analogs would lead to an increase of their anticancer activity and selectivity towards resistant cancer cells. The analogs with the 3-ketone-4,6-diene backbone show the highest apoptosis-inducing efficacy. The most potent compound, analog 406, possesses higher pro-apoptotic activity in chemo-resistant cell lines MCF-7TN-R and NCI/ADR-RES than the corresponding chemo-sensitive cell lines MCF-7 and OVCAR-8, respectively. However, this compound shows the same potency in inhibiting the growth of another pair of chemo-sensitive and chemo-resistant cancer cells, MCF-7 and MCF-7/Dox. Mechanism investigations indicate that analog 406 can induce apoptosis in chemo-resistant cancer cells through the mitochondrial pathway. Cellular glucosylceramide synthase assay shows that analog 406 does not interrupt glucosylceramide synthase in chemo-resistant cancer cell NCI/ADR-RES. These findings suggest that due to certain intrinsic properties, ceramide analogs' pro-apoptotic activity is not disrupted by the normal drug-resistance mechanisms, leading to their potential use for overcoming cancer multidrug-resistance.
Keywords: Anti-cancer drugs; Ceramide; Glucosylceramide synthase (GCS); Multidrug resistance; P-glycoprotein.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
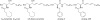

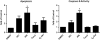


Similar articles
-
Novel ceramide analogs as potential chemotherapeutic agents in breast cancer.J Pharmacol Exp Ther. 2004 May;309(2):523-32. doi: 10.1124/jpet.103.062760. Epub 2004 Jan 23. J Pharmacol Exp Ther. 2004. PMID: 14742741
-
Ceramide glycosylation potentiates cellular multidrug resistance.FASEB J. 2001 Mar;15(3):719-30. doi: 10.1096/fj.00-0223com. FASEB J. 2001. PMID: 11259390
-
P-glycoprotein antagonists confer synergistic sensitivity to short-chain ceramide in human multidrug-resistant cancer cells.Exp Cell Res. 2011 Jul 15;317(12):1736-45. doi: 10.1016/j.yexcr.2011.03.004. Epub 2011 Mar 21. Exp Cell Res. 2011. PMID: 21396934 Free PMC article.
-
A review of ceramide analogs as potential anticancer agents.Future Med Chem. 2013 Aug;5(12):1405-21. doi: 10.4155/fmc.13.107. Future Med Chem. 2013. PMID: 23919551 Free PMC article. Review.
-
Ceramide glycosylation catalyzed by glucosylceramide synthase and cancer drug resistance.Adv Cancer Res. 2013;117:59-89. doi: 10.1016/B978-0-12-394274-6.00003-0. Adv Cancer Res. 2013. PMID: 23290777 Free PMC article. Review.
Cited by
-
Ceramide species are elevated in human breast cancer and are associated with less aggressiveness.Oncotarget. 2018 Apr 13;9(28):19874-19890. doi: 10.18632/oncotarget.24903. eCollection 2018 Apr 13. Oncotarget. 2018. PMID: 29731990 Free PMC article.
-
Anti-cancer effectiveness of a novel ceramide analog on chemo-sensitive and chemo-resistant breast cancers.Anticancer Drugs. 2024 Jan 1;35(1):12-21. doi: 10.1097/CAD.0000000000001536. Epub 2023 Aug 15. Anticancer Drugs. 2024. PMID: 37578744 Free PMC article.
-
Anticancer Effects of New Ceramides Isolated from the Red Sea Red Algae Hypnea musciformis in a Model of Ehrlich Ascites Carcinoma: LC-HRMS Analysis Profile and Molecular Modeling.Mar Drugs. 2022 Jan 10;20(1):63. doi: 10.3390/md20010063. Mar Drugs. 2022. PMID: 35049918 Free PMC article.
-
Therapeutic Potential of Ceramide in Cancer Treatment.J Cancer Res Oncobiol. 2024;4(1):134. Epub 2024 Sep 30. J Cancer Res Oncobiol. 2024. PMID: 40384804 Free PMC article.
-
Developing new ceramide analogs against non-small cell lung cancer (NSCLC).Am J Cancer Res. 2024 Jan 15;14(1):86-96. doi: 10.62347/AOGP2839. eCollection 2024. Am J Cancer Res. 2024. PMID: 38323290 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

