Complex self-assembly of pyrimido[4,5-d]pyrimidine nucleoside supramolecular structures
- PMID: 24457545
- PMCID: PMC3916841
- DOI: 10.1038/ncomms4108
Complex self-assembly of pyrimido[4,5-d]pyrimidine nucleoside supramolecular structures
Abstract
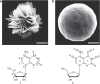
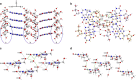
Supramolecular self-assembly is not only one of the chemical roots of biological structure but is also drawing attention in different industrial fields. Here we study the mechanism of the formation of a complex flower-shaped supramolecular structure of pyrimido[4,5-d]pyrimidine nucleosides by dynamic light scattering, scanning electron microscopy, differential scanning calorimetry, nuclear magnetic resonance and X-ray analysis. Upon removing the hydroxyl group of sugars, different flower-shaped superstructures can be produced. These works demonstrate that complex self-assembly can indeed be attained through hierarchical non-covalent interactions of single molecules. Furthermore, chimerical structures built from molecular recognition by these monomers indicate their potential in other fields if combined with other chemical entities.
Figures




References
-
- Buck M. R., Bondi J. F. & Schaak R. E. A total-synthesis framework for the construction of hihg-order colloidal hybrid nanoparticles. Nat. Chem. 4, 37–44 (2012). - PubMed
-
- Langille M. R., Zhang J., Personick M. L., Li S. & Mirkin C. A. Stepwise evolution of spherical seeds into 20-fold twinned icosahedra. Science 337, 954–957 (2012). - PubMed
-
- Lehn J. M. Perspectives in chemistry-steps towards complex matter. Angew. Chem. Int. Ed. 52, 2836–2850 (2013). - PubMed
-
- Jones M. R. & Mirkin C. A. Self-assembly gets new direction. Nature 491, 42–43 (2012). - PubMed
-
- Li G. L., Mohwald H. & Shchukin D. G. Precipitation polymerization for fabrication of complex core-shell hybrid particles and hollow structures. Chem. Soc. Rev. 42, 3628–3646 (2013). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

