Modular adeno-associated virus (rAAV) vectors used for cellular virus-directed enzyme prodrug therapy
- PMID: 24457557
- PMCID: PMC3901000
- DOI: 10.1038/srep03759
Modular adeno-associated virus (rAAV) vectors used for cellular virus-directed enzyme prodrug therapy
Abstract
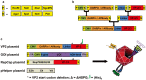
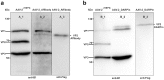
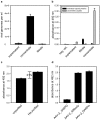
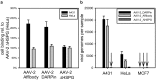
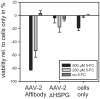
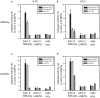
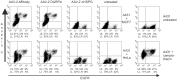
The pre-clinical and clinical development of viral vehicles for gene transfer increased in recent years, and a recombinant adeno-associated virus (rAAV) drug took center stage upon approval in the European Union. However, lack of standardization, inefficient purification methods and complicated retargeting limit general usability. We address these obstacles by fusing rAAV-2 capsids with two modular targeting molecules (DARPin or Affibody) specific for a cancer cell-surface marker (EGFR) while simultaneously including an affinity tag (His-tag) in a surface-exposed loop. Equipping these particles with genes coding for prodrug converting enzymes (thymidine kinase or cytosine deaminase) we demonstrate tumor marker specific transduction and prodrug-dependent apoptosis of cancer cells. Coding terminal and loop modifications in one gene enabled specific and scalable purification. Our genetic parts for viral production adhere to a standardized cloning strategy facilitating rapid prototyping of virus directed enzyme prodrug therapy (VDEPT).
Figures
Similar articles
-
Persistent anti-tumor effects via recombinant adeno-associated virus encoding herpes thymidine kinase gene monitored by PET-imaging.Oncol Rep. 2011 May;25(5):1263-9. doi: 10.3892/or.2011.1190. Epub 2011 Feb 17. Oncol Rep. 2011. PMID: 21331450
-
Novel strategy for generation and titration of recombinant adeno-associated virus vectors.J Virol. 2005 Jan;79(1):193-201. doi: 10.1128/JVI.79.1.193-201.2005. J Virol. 2005. PMID: 15596815 Free PMC article.
-
Gene-directed enzyme prodrug therapy for localized chemotherapeutics in allograft and xenograft tumor models.Cancer Gene Ther. 2014 Oct;21(10):434-40. doi: 10.1038/cgt.2014.47. Epub 2014 Sep 19. Cancer Gene Ther. 2014. PMID: 25236494 Free PMC article.
-
Recent developments in gene-directed enzyme prodrug therapy (GDEPT) for cancer.Curr Opin Mol Ther. 1999 Aug;1(4):480-6. Curr Opin Mol Ther. 1999. PMID: 11713763 Review.
-
Tissue and cell-type-specific transduction using rAAV vectors in lung diseases.J Mol Med (Berl). 2021 Aug;99(8):1057-1071. doi: 10.1007/s00109-021-02086-y. Epub 2021 May 21. J Mol Med (Berl). 2021. PMID: 34021360 Review.
Cited by
-
Genetically Encoded Self-Assembling Protein Nanoparticles for the Targeted Delivery In Vitro and In Vivo.Pharmaceutics. 2023 Jan 10;15(1):231. doi: 10.3390/pharmaceutics15010231. Pharmaceutics. 2023. PMID: 36678860 Free PMC article. Review.
-
Programmable Assembly of Adeno-Associated Virus-Antibody Composites for Receptor-Mediated Gene Delivery.Bioconjug Chem. 2020 Apr 15;31(4):1093-1106. doi: 10.1021/acs.bioconjchem.9b00790. Epub 2019 Dec 20. Bioconjug Chem. 2020. PMID: 31809024 Free PMC article.
-
Adeno-associated virus capsid protein expression in Escherichia coli and chemically defined capsid assembly.Sci Rep. 2019 Dec 9;9(1):18631. doi: 10.1038/s41598-019-54928-y. Sci Rep. 2019. PMID: 31819093 Free PMC article.
-
EGFR-Binding Peptides: From Computational Design towards Tumor-Targeting of Adeno-Associated Virus Capsids.Int J Mol Sci. 2020 Dec 15;21(24):9535. doi: 10.3390/ijms21249535. Int J Mol Sci. 2020. PMID: 33333826 Free PMC article.
-
Spatiotemporally confined red light-controlled gene delivery at single-cell resolution using adeno-associated viral vectors.Sci Adv. 2021 Jun 16;7(25):eabf0797. doi: 10.1126/sciadv.abf0797. Print 2021 Jun. Sci Adv. 2021. PMID: 34134986 Free PMC article.
References
-
- Springer C. J. & Niculescu-Duvaz I. Gene-directed enzyme prodrug therapy (GDEPT): choice of prodrugs. Adv. Drug Deliv. Rev. 22, 351–364 (1996). - PubMed
-
- Bhatia S. et al. Innovative Approaches for Enhancing Cancer Gene Therapy. Discov. Med. 15, 309–317 (2013). - PubMed
-
- Erbs P. et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 60, 3813–22 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

