Separation of photo-induced radical pair in cryptochrome to a functionally critical distance
- PMID: 24457842
- PMCID: PMC4894384
- DOI: 10.1038/srep03845
Separation of photo-induced radical pair in cryptochrome to a functionally critical distance
Abstract
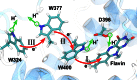
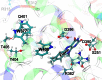
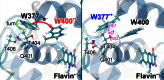
Cryptochrome is a blue light receptor that acts as a sensor for the geomagnetic field and assists many animals in long-range navigation. The magnetoreceptor function arises from light-induced formation of a radical pair through electron transfer between a flavin cofactor (FAD) and a triad of tryptophan residues. Here, this electron transfer is investigated by quantum chemical and classical molecular dynamics calculations. The results reveal how sequential electron transfer, assisted by rearrangement of polar side groups in the cryptochrome interior, can yield a FAD-Trp radical pair state with the FAD and Trp partners separated beyond a critical distance. The large radical pair separation reached establishes cryptochrome's sensitivity to the geomagnetic field through weakening of distance-dependent exchange and dipole-dipole interactions. It is estimated that the key secondary electron transfer step can overcome in speed both recombination (electron back-transfer) and proton transfer involving the radical pair reached after primary electron transfer.
Figures



Similar articles
-
Alternative radical pairs for cryptochrome-based magnetoreception.J R Soc Interface. 2014 Mar 26;11(95):20131063. doi: 10.1098/rsif.2013.1063. Print 2014 Jun 6. J R Soc Interface. 2014. PMID: 24671932 Free PMC article.
-
Origin of light-induced spin-correlated radical pairs in cryptochrome.J Phys Chem B. 2010 Nov 18;114(45):14745-54. doi: 10.1021/jp103401u. Epub 2010 Aug 4. J Phys Chem B. 2010. PMID: 20684534 Free PMC article.
-
Electron transfer and spin dynamics of the radical-pair in the cryptochrome from Chlamydomonas reinhardtii by computational analysis.J Chem Phys. 2020 Feb 14;152(6):065101. doi: 10.1063/1.5133019. J Chem Phys. 2020. PMID: 32061221
-
Photochemistry of flavoprotein light sensors.Nat Chem Biol. 2014 Oct;10(10):801-9. doi: 10.1038/nchembio.1633. Nat Chem Biol. 2014. PMID: 25229449 Free PMC article. Review.
-
Photolyase: Dynamics and electron-transfer mechanisms of DNA repair.Arch Biochem Biophys. 2017 Oct 15;632:158-174. doi: 10.1016/j.abb.2017.08.007. Epub 2017 Aug 9. Arch Biochem Biophys. 2017. PMID: 28802828 Free PMC article. Review.
Cited by
-
Diamond surface engineering for molecular sensing with nitrogen-vacancy centers.J Mater Chem C Mater. 2022 Sep 1;10(37):13533-13569. doi: 10.1039/d2tc01258h. eCollection 2022 Sep 29. J Mater Chem C Mater. 2022. PMID: 36324301 Free PMC article. Review.
-
Molecular Insights into Variable Electron Transfer in Amphibian Cryptochrome.Biophys J. 2018 Jun 5;114(11):2563-2572. doi: 10.1016/j.bpj.2018.04.014. Biophys J. 2018. PMID: 29874607 Free PMC article.
-
How Far Does a Receptor Influence Vibrational Properties of an Odorant?PLoS One. 2016 Mar 25;11(3):e0152345. doi: 10.1371/journal.pone.0152345. eCollection 2016. PLoS One. 2016. PMID: 27014869 Free PMC article.
-
Weak Broadband Electromagnetic Fields are More Disruptive to Magnetic Compass Orientation in a Night-Migratory Songbird (Erithacus rubecula) than Strong Narrow-Band Fields.Front Behav Neurosci. 2016 Mar 22;10:55. doi: 10.3389/fnbeh.2016.00055. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27047356 Free PMC article.
-
Condensed Matter Systems Exposed to Radiation: Multiscale Theory, Simulations, and Experiment.Chem Rev. 2024 Jul 10;124(13):8014-8129. doi: 10.1021/acs.chemrev.3c00902. Epub 2024 Jun 6. Chem Rev. 2024. PMID: 38842266 Free PMC article. Review.
References
-
- Ball P. Physics of life: The dawn of quantum biology. Nature 474, 272–274 (2011). - PubMed
-
- Wiltschko W. & Wiltschko R. Magnetic compass of European robins. Science 176, 62–64 (1972). - PubMed
-
- Hein C. M., Engels S., Kishkinev D. & Mouritsen H. Robins have a magnetic compass in both eyes. Nature 471, E11–E12 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

