Interaction between the ADAMTS-12 metalloprotease and fibulin-2 induces tumor-suppressive effects in breast cancer cells
- PMID: 24457941
- PMCID: PMC4012729
- DOI: 10.18632/oncotarget.1690
Interaction between the ADAMTS-12 metalloprotease and fibulin-2 induces tumor-suppressive effects in breast cancer cells
Abstract
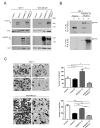
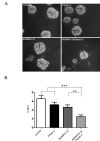
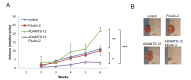
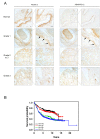
Balance between pro-tumor and anti-tumor effects may be affected by molecular interactions within tumor microenvironment. On this basis we searched for molecular partners of ADAMTS-12, a secreted metalloprotease that shows both oncogenic and tumor-suppressive effects. Using its spacer region as a bait in a yeast two-hybrid screen, we identified fibulin-2 as a potential ADAMTS-12-interacting protein. Fibulins are components of basement membranes and elastic matrix fibers in connective tissue. Besides this structural function, fibulins also play crucial roles in different biological events, including tumorigenesis. To examine the functional consequences of the ADAMTS-12/fibulin-2 interaction, we performed different in vitro assays using two breast cancer cell lines: the poorly invasive MCF-7 and the highly invasive MDA-MB-231. Overall our data indicate that this interaction promotes anti-tumor effects in breast cancer cells. To assess the in vivo relevance of this interaction, we induced tumors in nude mice using MCF-7 cells expressing both ADAMTS-12 and fibulin-2 that showed a remarkable growth deficiency. Additionally, we also found that ADAMTS-12 may elicit pro-tumor effects in the absence of fibulin-2. Immunohistochemical staining of breast cancer samples allowed the detection of both ADAMTS-12 and fibulin-2 in the connective tissue surrounding tumor area in less aggressive carcinomas. However, both proteins are hardly detected in more aggressive tumors. These data and survival analysis plots of breast cancer patients suggest that concomitant detection of ADAMTS-12 and fibulin-2 could be a good prognosis marker in breast cancer diagnosis.
Figures


Similar articles
-
Cleavage of Fibulin-2 by the aggrecanases ADAMTS-4 and ADAMTS-5 contributes to the tumorigenic potential of breast cancer cells.Oncotarget. 2017 Feb 21;8(8):13716-13729. doi: 10.18632/oncotarget.14627. Oncotarget. 2017. PMID: 28099917 Free PMC article.
-
Fibulin-1 interacts with Sex Hormone Binding Globulin and is linked to less aggressive estrogen-dependent breast cancers.Life Sci. 2018 Aug 15;207:372-380. doi: 10.1016/j.lfs.2018.06.024. Epub 2018 Jun 22. Life Sci. 2018. PMID: 29940241
-
Expression of extracellular matrix proteins fibulin-1 and fibulin-2 by human corneal fibroblasts.Curr Eye Res. 2007 Jun;32(6):481-90. doi: 10.1080/02713680701411269. Curr Eye Res. 2007. PMID: 17612964
-
ADAMTS proteases and cancer.Matrix Biol. 2015 May-Jul;44-46:77-85. doi: 10.1016/j.matbio.2015.01.013. Epub 2015 Jan 28. Matrix Biol. 2015. PMID: 25636539 Review.
-
Roles of Fibulin-2 in Carcinogenesis.Med Sci Monit. 2020 Jan 9;26:e918099. doi: 10.12659/MSM.918099. Med Sci Monit. 2020. PMID: 31915327 Free PMC article. Review.
Cited by
-
Methylation of MGMT and ADAMTS14 in normal colon mucosa: biomarkers of a field defect for cancerization preferentially targeting elder African-Americans.Oncotarget. 2015 Feb 20;6(5):3420-31. doi: 10.18632/oncotarget.2852. Oncotarget. 2015. PMID: 25638164 Free PMC article.
-
ADAMTS16 mutations sensitize ovarian cancer cells to platinum-based chemotherapy.Oncotarget. 2016 Aug 8;8(51):88410-88420. doi: 10.18632/oncotarget.11120. eCollection 2017 Oct 24. Oncotarget. 2016. PMID: 29179445 Free PMC article.
-
Fibulin-2 expression associates with vascular invasion and patient survival in breast cancer.PLoS One. 2021 Apr 9;16(4):e0249767. doi: 10.1371/journal.pone.0249767. eCollection 2021. PLoS One. 2021. PMID: 33836007 Free PMC article.
-
A Novel Quantification System Combining iTRAQ Technology and Multi-Omics Assessment to Predict Prognosis and Immunotherapy Efficacy in Colon Cancer.Front Bioeng Biotechnol. 2022 Apr 4;10:862619. doi: 10.3389/fbioe.2022.862619. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35445008 Free PMC article.
-
AK001058 promotes the proliferation and migration of colorectal cancer cells by regulating methylation of ADAMTS12.Am J Transl Res. 2019 Sep 15;11(9):5869-5878. eCollection 2019. Am J Transl Res. 2019. PMID: 31632555 Free PMC article.
References
-
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. - PubMed
-
- Happonen KE, Heinegard D, Saxne T, Blom AM. Interactions of the complement system with molecules of extracellular matrix: relevance for joint diseases. Immunobiology. 2012;217(11):1088–1096. - PubMed
-
- Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4(6):479–489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

