Distinct features in natural history and outcomes of acute hepatitis C
- PMID: 24457946
- PMCID: PMC4112167
- DOI: 10.1097/MCG.0000000000000076
Distinct features in natural history and outcomes of acute hepatitis C
Abstract
Background: Acute hepatitis C (AHCV) provides a diagnostic challenge with diverse clinical presentations.
Goals: This study was aimed to examine the clinical and demographic features as well as outcomes in AHCV patients identified from inpatient and outpatient hospital settings.
Study: Patients with suspected AHCV were recruited from Philadelphia VA Medical Center, Hospital of University of Pennsylvania and Brooklyn VA Medical Center between 2000 and 2010. AHCV was diagnosed by acute serum alanine aminotransferase elevation with anti-hepatitis C virus (HCV) seroconversion, HCV-RNA fluctuations above 1 log, and/or recent high-risk exposure without prior HCV infection, excluding those with human immunodeficiency virus infection. Clinical and therapeutic outcomes were monitored for at least 6 months.
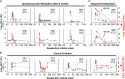
Results: A total of 40 AHCV patients were enrolled with a median follow-up of 129 weeks. They were mostly men (68%) and whites (73%) with median age of 43 years, diverse risk factors (33% injection drugs, 20% health care-associated, 3% sexual, and 45% unknown), and wide variations in peak alanine aminotransferase (143 to 3435 U/L) and total bilirubin levels (0.4 to 19.3 mg/dL). Viremia resolved spontaneously in 23% and persisted without therapy in 27%, whereas 50% received interferon α-based therapy with 90% cure (18/20). Distinct clinical scenarios included: (1) wide viremic fluctuations >1 log (65%) and intermittent HCV-RNA negativity; (2) autoantibodies (25% antinuclear antibodies, 69% antismooth muscle antibodies) or autoimmune features; (3) delayed spontaneous viral clearance in 2 patients; (4) rapid cirrhosis progression in 2 patients.
Conclusions: AHCV is a heterogenous disease that requires careful monitoring. The lack of apparent risk factor in high proportion of patients and its diverse presentations warrant diagnostic vigilance.
Conflict of interest statement
The authors declare that they have nothing to disclose.
Figures


References
-
- Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol. 2008;103:1283–1297quiz 1298. - PubMed
-
- Morin T, Pariente A, Lahmek P, et al. Acute hepatitis C: analysis of a 126-case prospective, multicenter cohort. Eur J Gastroenterol Hepatol. 2010;22:157–166. - PubMed
-
- Santantonio T, Medda E, Ferrari C, et al. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154–1159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

