Stress-induced RNASET2 overexpression mediates melanocyte apoptosis via the TRAF2 pathway in vitro
- PMID: 24457966
- PMCID: PMC4040706
- DOI: 10.1038/cddis.2013.539
Stress-induced RNASET2 overexpression mediates melanocyte apoptosis via the TRAF2 pathway in vitro
Abstract
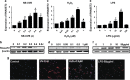
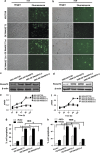
The recent genome-wide association study identified a link between vitiligo and genetic variants in the ribonuclease T2 (RNASET2) gene; however, the functional roles of RNASET2 in vitiligo pathogenesis or in melanocyte apoptosis have yet to be determined. The current study was designed to investigate the vitiligo-related expression pattern of RNASET2 and its molecular function involving apoptosis-related signaling proteins and pathways. The results showed overexpression of RNASET2 in epidermis specimens from 40 vitiligo patients compared with that from matched healthy controls. In addition, in vitro analyses indicated that overexpression of RNASET2 was inducible in cultured primary human melanocytes and keratinocytes by stress conditions, that is, exposure to UV irradiation, hydrogen peroxide, and inflammatory factors, respectively, and led to increased cell apoptosis via the tumor necrosis factor receptor-associated factor 2 (TRAF2)-caspases pathway through the physical interaction of RNASET2 with TRAF2. Thus, RNASET2 may contribute to vitiligo pathogenesis by inhibiting TRAF2 expression and, as such, RNASET2 may represent a potential therapeutic target of vitiligo.
Figures






References
-
- Taieb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med. 2009;360:160–169. - PubMed
-
- Schaffer JV, Bolognia JL. The treatment of hypopigmentation in children. Clin Dermatol. 2003;21:296–310. - PubMed
-
- Westerhof W, D'Ischia M. Vitiligo puzzle: the pieces fall in place. Pigment Cell Res. 2007;20:345–359. - PubMed
-
- Quan C, Ren YQ, Xiang LH, Sun LD, Xu AE, Gao XH, et al. Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat Genet. 2010;42:614–618. - PubMed
-
- Acquati F, Nucci C, Bianchi MG, Gorletta T, Taramelli R. Molecular cloning, tissue distribution, and chromosomal localization of the human homolog of the R2/Th/Stylar ribonuclease gene family. Methods Mol Biol. 2001;160:87–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

