Upregulation of KCNQ1/KCNE1 K+ channels by Klotho
- PMID: 24457979
- PMCID: PMC4203751
- DOI: 10.4161/chan.27662
Upregulation of KCNQ1/KCNE1 K+ channels by Klotho
Abstract
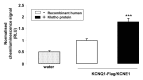
Klotho is a transmembrane protein expressed primarily in kidney, parathyroid gland, and choroid plexus. The extracellular domain could be cleaved off and released into the systemic circulation. Klotho is in part effective as β-glucuronidase regulating protein stability in the cell membrane. Klotho is a major determinant of aging and life span.Overexpression of Klotho increases and Klotho deficiency decreases life span. Klotho deficiency may further result in hearing loss and cardiac arrhythmia. The present study explored whether Klotho modifies activity and protein abundance of KCNQ1/KCNE1, a K(+) channel required for proper hearing and cardiac repolarization. To this end, cRNA encoding KCNQ1/KCNE1 was injected in Xenopus oocytes with or without additional injection of cRNA encoding Klotho. KCNQ1/KCNE1 expressing oocytes were treated with human recombinant Klotho protein (30 ng/mL) for 24 h. Moreover, oocytes which express both KCNQ1/KCNE1 and Klotho were treated with 10 μM DSA L (D-saccharic acid-1,4-lactone), a β-glucuronidase inhibitor. The KCNQ1/KCNE1 depolarization-induced current (I(Ks)) was determined utilizing dual electrode voltage clamp, while KCNQ1/KCNE1 protein abundance in the cell membrane was visualized utilizing specific antibody binding and quantified by chemiluminescence. KCNQ1/KCNE1 channel activity and KCNQ1/KCNE1 protein abundance were upregulated by coexpression of Klotho. The effect was mimicked by treatment with human recombinant Klotho protein (30 ng/mL) and inhibited by DSA L (10 μM). In conclusion, Klotho upregulates KCNQ1/KCNE1 channel activity by “mainly” enhancing channel protein abundance in the plasma cell membrane, an effect at least partially mediated through the β-glucuronidase activity of Klotho protein.
Figures




Comment in
-
Klotho: a new trafficking modifier of Kv7.1/KCNE1 channels.Channels (Austin). 2014;8(4):285. doi: 10.4161/chan.29659. Channels (Austin). 2014. PMID: 25478618 Free PMC article. No abstract available.
References
-
- Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–82. doi: 10.1161/01.CIR.0000124224.48962.32. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
