Glial cell line-derived neurotrophic factor protects against high-fat diet-induced obesity
- PMID: 24458024
- PMCID: PMC3949027
- DOI: 10.1152/ajpgi.00364.2013
Glial cell line-derived neurotrophic factor protects against high-fat diet-induced obesity
Abstract
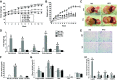
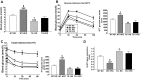
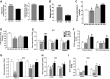
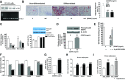
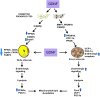
Obesity is a growing epidemic with limited effective treatments. The neurotrophic factor glial cell line-derived neurotrophic factor (GDNF) was recently shown to enhance β-cell mass and improve glucose control in rodents. Its role in obesity is, however, not well characterized. In this study, we investigated the ability of GDNF to protect against high-fat diet (HFD)-induced obesity. GDNF transgenic (Tg) mice that overexpress GDNF under the control of the glial fibrillary acidic protein promoter and wild-type (WT) littermates were maintained on a HFD or regular rodent diet for 11 wk, and weight gain, energy expenditure, and insulin sensitivity were monitored. Differentiated mouse brown adipocytes and 3T3-L1 white adipocytes were used to study the effects of GDNF in vitro. Tg mice resisted the HFD-induced weight gain, insulin resistance, dyslipidemia, hyperleptinemia, and hepatic steatosis seen in WT mice despite similar food intake and activity levels. They exhibited significantly (P<0.001) higher energy expenditure than WT mice and increased expression in skeletal muscle and brown adipose tissue of peroxisome proliferator activated receptor-α and β1- and β3-adrenergic receptor genes, which are associated with increased lipolysis and enhanced lipid β-oxidation. In vitro, GDNF enhanced β-adrenergic-mediated cAMP release in brown adipocytes and suppressed lipid accumulation in differentiated 3T3L-1 cells through a p38MAPK signaling pathway. Our studies demonstrate a novel role for GDNF in the regulation of high-fat diet-induced obesity through increased energy expenditure. They show that GDNF and its receptor agonists may be potential targets for the treatment or prevention of obesity.
Keywords: beta-oxidation; energy expenditure; hepatic steatosis; neurotrophic; β-adrenergic signaling.
Figures


References
-
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409: 729–733, 2001 - PubMed
-
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394, 2002 - PubMed
-
- Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet 21: 76–83, 1999 - PubMed
-
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002 - PubMed
-
- Bronnikov G, Bengtsson T, Kramarova L, Golozoubova V, Cannon B, Nedergaard J. β1 to β3 switch in control of cyclic adenosine monophosphate during brown adipocyte development explains distinct beta-adrenoceptor subtype mediation of proliferation and differentiation. Endocrinology 140: 4185–4197, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

