A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with CKD
- PMID: 24458078
- PMCID: PMC3974353
- DOI: 10.2215/CJN.05320513
A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with CKD
Abstract
Background and objectives: Few randomized controlled trials have compared intravenous iron products head to head in CKD patients with iron deficiency anemia. This study compared the efficacy and safety of two intravenous iron products (ferumoxytol [Feraheme injection] and iron sucrose [Venofer]) in patients with CKD and iron deficiency anemia.
Design, setting, participants, & measurements: In this phase II, randomized, open-label, active-controlled, multicenter clinical trial, patients were randomized 1:1 to either 1.02 g ferumoxytol (2 × 510-mg injections) or 1.0 g iron sucrose administered as either a slow injection or infusion (10 doses for dialysis patients and 5 doses for nondialysis patients). Inclusion criteria included hemoglobin<11.0 g/dl, transferrin saturation<30%, and eGFR<60 ml/min per 1.73 m(2) or a diagnosis of underlying CKD (e.g., nephropathy or nephritis). The primary end point was change in hemoglobin from baseline to week 5.
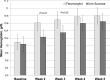
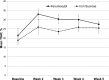
Results: In total, 162 patients were randomized. Demographics were balanced between the treatment groups. Adverse event profiles of the two regimens were fairly similar: overall adverse events, 48% ferumoxytol versus 65% iron sucrose; related adverse events, 10% ferumoxytol versus 16% iron sucrose; and adverse events leading to study discontinuation, 1% ferumoxytol versus 5% iron sucrose. Rates of serious adverse events and related serious adverse events were similar between the ferumoxytol and iron sucrose groups: serious adverse events, 9% versus 7%, respectively and related serious adverse events, 1% versus 1%, respectively. Overall, increases in hemoglobin were similar between treatment groups. Based on an ANOVA model adjusted for baseline hemoglobin level and dialysis status, the least squares mean change from baseline to week 5 was 0.8 ± 0.1 g/dl in the ferumoxytol-treated group and 0.7 ± 0.1 g/dl in the iron sucrose group. The difference in the mean change from baseline between the two treatment groups was 0.1 g/dl (95% confidence interval, -0.2 to 0.4).
Conclusion: In this randomized, controlled trial, ferumoxytol and iron sucrose showed comparable efficacy and adverse events rates.
Trial registration: ClinicalTrials.gov NCT01052779.
Figures

References
-
- Valderrábano F, Hörl WH, Macdougall IC, Rossert J, Rutkowski B, Wauters JP: Pre-dialysis survey on anaemia management. Nephrol Dial Transplant 18: 89–100, 2003 - PubMed
-
- Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, Pereira BJ: Anemia: An early complication of chronic renal insufficiency. Am J Kidney Dis 38: 803–812, 2001 - PubMed
-
- KDOQI. National Kidney Foundation : KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis 47[5 Suppl 3]: S1–S146, 2006 - PubMed
-
- Kidney Disease : Improving Global Outcomes (KDIGO) Anemia Work Group: KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int 2: 279–331, 2012
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

