Glycogen synthase kinase-3β inhibition ameliorates cardiac parasympathetic dysfunction in type 1 diabetic Akita mice
- PMID: 24458356
- PMCID: PMC4030105
- DOI: 10.2337/db12-1459
Glycogen synthase kinase-3β inhibition ameliorates cardiac parasympathetic dysfunction in type 1 diabetic Akita mice
Abstract
Decreased heart rate variability (HRV) is a major risk factor for sudden death and cardiovascular disease. We previously demonstrated that parasympathetic dysfunction in the heart of the Akita type 1 diabetic mouse was due to a decrease in the level of the sterol response element-binding protein (SREBP-1). Here we demonstrate that hyperactivity of glycogen synthase kinase-3β (GSK3β) in the atrium of the Akita mouse results in decreased SREBP-1, attenuation of parasympathetic modulation of heart rate, measured as a decrease in the high-frequency (HF) fraction of HRV in the presence of propranolol, and a decrease in expression of the G-protein coupled inward rectifying K(+) (GIRK4) subunit of the acetylcholine (ACh)-activated inward-rectifying K(+) channel (IKACh), the ion channel that mediates the heart rate response to parasympathetic stimulation. Treatment of atrial myocytes with the GSK3β inhibitor Kenpaullone increased levels of SREBP-1 and expression of GIRK4 and IKACh, whereas a dominant-active GSK3β mutant decreased SREBP-1 and GIRK4 expression. In Akita mice treated with GSK3β inhibitors Li(+) and/or CHIR-99021, Li(+) increased IKACh, and Li(+) and CHIR-99021 both partially reversed the decrease in HF fraction while increasing GIRK4 and SREBP-1 expression. These data support the conclusion that increased GSK3β activity in the type 1 diabetic heart plays a critical role in parasympathetic dysfunction through an effect on SREBP-1, supporting GSK3β as a new therapeutic target for diabetic autonomic neuropathy.
© 2014 by the American Diabetes Association.
Figures




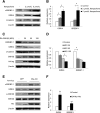
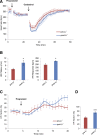
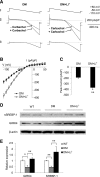
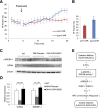
Comment in
-
Complexity of impaired parasympathetic heart rate regulation in diabetes.Diabetes. 2014 Jun;63(6):1847-9. doi: 10.2337/db14-0304. Diabetes. 2014. PMID: 24853900 No abstract available.
References
-
- Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med 1997;126:296–306 - PubMed
-
- Brown DW, Giles WH, Greenlund KJ, Valdez R, Croft JB. Impaired fasting glucose, diabetes mellitus, and cardiovascular disease risk factors are associated with prolonged QTc duration. Results from the Third National Health and Nutrition Examination Survey. J Cardiovasc Risk 2001;8:227–233 - PubMed
-
- Allessie MA, Lammers WJ, Bonke IM, Hollen J. Intra-atrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in the dog. Circulation 1984;70:123–135 - PubMed
-
- Aronson D. Pharmacologic modulation of autonomic tone: implications for the diabetic patient. Diabetologia 1997;40:476–481 - PubMed
-
- Valensi P, Paries J, Attali JR; French Group for Research and Study of Diabetic Neuropathy. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications–the French multicenter study. Metabolism 2003;52:815–820 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

