Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity
- PMID: 24458645
- PMCID: PMC4313730
- DOI: 10.1126/science.1247606
Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity
Abstract
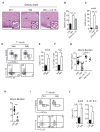
How the immune system adapts to malnutrition to sustain immunity at barrier surfaces, such as the intestine, remains unclear. Vitamin A deficiency is one of the most common micronutrient deficiencies and is associated with profound defects in adaptive immunity. Here, we found that type 3 innate lymphoid cells (ILC3s) are severely diminished in vitamin A-deficient settings, which results in compromised immunity to acute bacterial infection. However, vitamin A deprivation paradoxically resulted in dramatic expansion of interleukin-13 (IL-13)-producing ILC2s and resistance to nematode infection in mice, which revealed that ILCs are primary sensors of dietary stress. Further, these data indicate that, during malnutrition, a switch to innate type 2 immunity may represent a powerful adaptation of the immune system to promote host survival in the face of ongoing barrier challenges.
Figures



Comment in
-
Innate lymphoid cells: nutrients direct immune balance.Nat Rev Immunol. 2014 Mar;14(3):137. doi: 10.1038/nri3626. Epub 2014 Feb 7. Nat Rev Immunol. 2014. PMID: 24503524 No abstract available.
References
-
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. - PubMed
-
- WHO. Soil-transmitted helminth infections (factsheet) World Health Organization; 2013.
-
- Kiss EA, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. - PubMed
-
- Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

