Hybrid phospholipid bilayer coatings for separations of cationic proteins in capillary zone electrophoresis
- PMID: 24459085
- PMCID: PMC4041483
- DOI: 10.1002/elps.201300537
Hybrid phospholipid bilayer coatings for separations of cationic proteins in capillary zone electrophoresis
Abstract
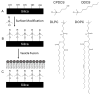
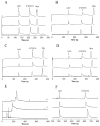
Protein separations in CZE suffer from nonspecific adsorption of analytes to the capillary surface. Semipermanent phospholipid bilayers have been used to minimize adsorption, but must be regenerated regularly to ensure reproducibility. We investigated the formation, characterization, and use of hybrid phospholipid bilayers (HPBs) as more stable biosurfactant capillary coatings for CZE protein separations. HPBs are formed by covalently modifying a support with a hydrophobic monolayer onto which a self-assembled lipid monolayer is deposited. Monolayers prepared in capillaries using 3-cyanopropyldimethylchlorosilane (CPDCS) or n-octyldimethylchlorosilane (ODCS) yielded hydrophobic surfaces with lowered surface free energies of 6.0 ± 0.3 or 0.2 ± 0.1 mJ m(-2) , respectively, compared to 17 ± 1 mJ m(-2) for bare silica capillaries. HPBs were formed by subsequently fusing vesicles comprised of 1,2-dilauroyl-sn-glycero-3-phosphocholine or 1,2-dioleoyl-sn-glycero-3-phosphocholine to CPDCS- or ODCS-modified capillaries. The resultant HPB coatings shielded the capillary surface and yielded reduced electroosmotic mobility (1.3-1.9 × 10(-4) cm(2) V(-1) s(-1) ) compared to CPDCS- and ODCS-modified or bare capillaries (3.6 ± 0.2 × 10(-4) cm(2) V(-1) s(-1) , 4.8 ± 0.4 × 10(-4) cm(2) V(-1) s(-1) , and 6.0 ± 0.2 × 10(-4) cm(2) V(-1) s(-1) , respectively), with increased stability compared to phospholipid bilayer coatings. HPB-coated capillaries yielded reproducible protein migration times (RSD ≤ 3.6%, n ≥ 6) with separation efficiencies as high as 200 000 plates/m.
Keywords: CZE; Capillary coatings; Hybrid bilayers; Phospholipids; Proteins.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors claim no financial or commercial conflicts of interest associated with this work.
Figures

Similar articles
-
Factors affecting the behavior and effectiveness of phospholipid bilayer coatings for capillary electrophoretic separations of basic proteins.Anal Chem. 2008 Mar 1;80(5):1806-12. doi: 10.1021/ac702408u. Epub 2008 Jan 31. Anal Chem. 2008. PMID: 18232711
-
Oligomerized phospholipid bilayers as semipermanent coatings in capillary electrophoresis.Anal Chem. 2005 Apr 1;77(7):2015-21. doi: 10.1021/ac0489622. Anal Chem. 2005. PMID: 15801732
-
Polymerized phospholipid bilayers as permanent coatings for small amine separations using mixed aqueous/organic capillary zone electrophoresis.J Chromatogr A. 2012 Dec 7;1267:80-8. doi: 10.1016/j.chroma.2012.07.017. Epub 2012 Jul 13. J Chromatogr A. 2012. PMID: 22854223
-
Metal cation control of electroosmotic flow magnitude in phospholipid-coated capillaries.Electrophoresis. 2016 May;37(10):1303-9. doi: 10.1002/elps.201600012. Epub 2016 Mar 31. Electrophoresis. 2016. PMID: 26960035
-
Non-covalent capillary coatings for protein separations in capillary electrophoresis.J Chromatogr A. 2008 Mar 14;1184(1-2):81-105. doi: 10.1016/j.chroma.2007.10.114. Epub 2007 Dec 4. J Chromatogr A. 2008. PMID: 18164023 Review.
Cited by
-
Methacrylate Polymer Scaffolding Enhances the Stability of Suspended Lipid Bilayers for Ion Channel Recordings and Biosensor Development.ACS Biomater Sci Eng. 2015;1(10):955-963. doi: 10.1021/acsbiomaterials.5b00205. ACS Biomater Sci Eng. 2015. PMID: 26925461 Free PMC article.
-
Recent advances in protein analysis by capillary and microchip electrophoresis.Analyst. 2017 May 30;142(11):1847-1866. doi: 10.1039/c7an00198c. Analyst. 2017. PMID: 28470231 Free PMC article. Review.
-
Highly stabilized, polymer-lipid membranes prepared on silica microparticles as stationary phases for capillary chromatography.J Chromatogr A. 2015 Mar 13;1385:28-34. doi: 10.1016/j.chroma.2015.01.052. Epub 2015 Jan 24. J Chromatogr A. 2015. PMID: 25670414 Free PMC article.
-
Hybrid bilayer membranes as platforms for biomimicry and catalysis.Nat Rev Chem. 2022 Dec;6(12):862-880. doi: 10.1038/s41570-022-00433-2. Epub 2022 Oct 28. Nat Rev Chem. 2022. PMID: 37117701 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

