Polycystin-1 negatively regulates Polycystin-2 expression via the aggresome/autophagosome pathway
- PMID: 24459142
- PMCID: PMC3945307
- DOI: 10.1074/jbc.M113.501205
Polycystin-1 negatively regulates Polycystin-2 expression via the aggresome/autophagosome pathway
Abstract
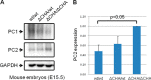
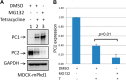
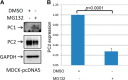
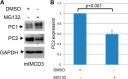
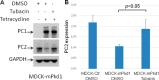
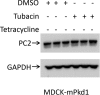
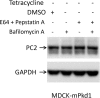
Mutations of the PKD1 and PKD2 genes, encoding polycystin-1 (PC1) and polycystin-2 (PC2), respectively, lead to autosomal dominant polycystic kidney disease. Interestingly, up-regulation or down-regulation of PKD1 or PKD2 leads to polycystic kidney disease in animal models, but their interrelations are not completely understood. We show here that full-length PC1 that interacts with PC2 via a C-terminal coiled-coil domain regulates PC2 expression in vivo and in vitro by down-regulating PC2 expression in a dose-dependent manner. Expression of the pathogenic mutant R4227X, which lacks the C-terminal coiled-coil domain, failed to down-regulate PC2 expression, suggesting that PC1-PC2 interaction is necessary for PC2 regulation. The proteasome and autophagy are two pathways that control protein degradation. Proteins that are not degraded by proteasomes precipitate in the cytoplasm and are transported via histone deacetylase 6 (HDAC6) toward the aggresomes. We found that HDAC6 binds to PC2 and that expression of full-length PC1 accelerates the transport of the HDAC6-PC2 complex toward aggresomes, whereas expression of the R4227X mutant fails to do so. Aggresomes are engulfed by autophagosomes, which then fuse with the lysosome for degradation; this process is also known as autophagy. We have now shown that PC1 overexpression leads to increased degradation of PC2 via autophagy. Interestingly, PC1 does not activate autophagy generally. Thus, we have now uncovered a new pathway suggesting that when PC1 is expressed, PC2 that is not bound to PC1 is directed to aggresomes and subsequently degraded via autophagy, a control mechanism that may play a role in autosomal dominant polycystic kidney disease pathogenesis.
Keywords: Calcium Channels; Physiology; Protein Degradation; Protein Processing; Trafficking.
Figures












References
-
- Hughes J., Ward C. J., Peral B., Aspinwall R., Clark K., San Millán J. L., Gamble V., Harris P. C. (1995) The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151–160 - PubMed
-
- Nims N., Vassmer D., Maser R. L. (2003) Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene. Evidence for 11 membrane-spanning domains. Biochemistry 42, 13035–13048 - PubMed
-
- Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A., Kimberling W. J., Breuning M. H., Deltas C. C., Peters D. J., Somlo S. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 - PubMed
-
- Qian F., Germino F. J., Cai Y., Zhang X., Somlo S., Germino G. G. (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 16, 179–183 - PubMed
-
- Hanaoka K., Qian F., Boletta A., Bhunia A. K., Piontek K., Tsiokas L., Sukhatme V. P., Guggino W. B., Germino G. G. (2000) Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990–994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

