Defining the functional boundaries of the murine α1,3-fucosyltransferase Fut7 reveals a remarkably compact locus
- PMID: 24459148
- PMCID: PMC3945301
- DOI: 10.1074/jbc.M113.511790
Defining the functional boundaries of the murine α1,3-fucosyltransferase Fut7 reveals a remarkably compact locus
Abstract
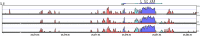
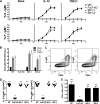
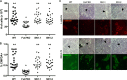
Fut7 encodes an α1,3-fucosyltransferase critical for biosynthesis of glycan ligands for all three selectins. Consistent with this function, Fut7 expression is limited to hematopoietic cells and high endothelial cells which express selectin ligands. Mechanisms that govern Fut7 expression are poorly defined. To begin to understand the molecular genetic basis for transcriptional regulation of Fut7, a transgenic, gain-of-function, genetic complementation approach in mice was used to define the "functional boundaries" of the murine Fut7 locus, defined here as any uninterupted stretch of genomic DNA that contains all cis-acting genetic elements essential for accurate physiologic expression. A 12.7-kb contiguous genomic interval, which lies completely between the highly conserved flanking Npdc1 and Abca2 loci on chromosome 2 and which contains the complete transcriptional unit plus ∼7.4 kb upstream of the transcriptional start site and ∼2 kb downstream of the transcriptional termination and polyadenylation sites, was used as a transgene (Tg) on a Fut7 null background. Tg+ mice exhibited restoration of Fut7 gene expression and physiologic levels of selectin ligand expression and function on neutrophils, activated T cells, and high endothelial cells and corrected the functional defects in these cells found in Fut7 null mice without leading to detectable expression of Fut7 in normally non-expressing tissues. These results demonstrate that all genetic information essential for appropriate and selective expression of Fut7 in diverse cell types and in response to distinct developmental signals is contained within this comparatively small genetic region.
Keywords: Cell Adhesion; Gene Expression; Glycobiology; Glycosyltransferases; Immunology; Leukocyte; Transcription Regulation.
Figures



References
-
- Gallatin W. M., Weissman I. L., Butcher E. C. (1983) A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 304, 30–34 - PubMed
-
- Lasky L. A., Singer M. S., Yednock T. A., Dowbenko D., Fennie C., Rodriguez H., Nguyen T., Stachel S., Rosen S. D. (1989) Cloning of a lymphocyte homing receptor reveals a lectin domain. Cell 56, 1045–1055 - PubMed
-
- Stoolman L. M., Yednock T. A., Rosen S. D. (1987) Homing receptors on human and rodent lymphocytes- evidence for a conserved carbohydrate-binding specificity. Blood 70, 1842–1850 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

