BK channel-mediated relaxation of urinary bladder smooth muscle: a novel paradigm for phosphodiesterase type 4 regulation of bladder function
- PMID: 24459245
- PMCID: PMC3965888
- DOI: 10.1124/jpet.113.210708
BK channel-mediated relaxation of urinary bladder smooth muscle: a novel paradigm for phosphodiesterase type 4 regulation of bladder function
Abstract
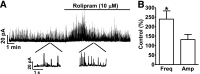
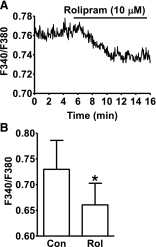
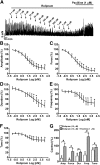
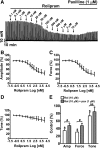
Elevation of intracellular cAMP and activation of protein kinase A (PKA) lead to activation of large conductance voltage- and Ca(2+)-activated K(+) (BK) channels, thus attenuation of detrusor smooth muscle (DSM) contractility. In this study, we investigated the mechanism by which pharmacological inhibition of cAMP-specific phosphodiesterase 4 (PDE4) with rolipram or Ro-20-1724 (C(15)H(22)N(2)O(3)) suppresses guinea pig DSM excitability and contractility. We used high-speed line-scanning confocal microscopy, ratiometric fluorescence Ca(2+) imaging, and perforated whole-cell patch-clamp techniques on freshly isolated DSM cells, along with isometric tension recordings of DSM isolated strips. Rolipram caused an increase in the frequency of Ca(2+) sparks and the spontaneous transient BK currents (TBKCs), hyperpolarized the cell membrane potential (MP), and decreased the intracellular Ca(2+) levels. Blocking BK channels with paxilline reversed the hyperpolarizing effect of rolipram and depolarized the MP back to the control levels. In the presence of H-89 [N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide dihydrochloride], a PKA inhibitor, rolipram did not cause MP hyperpolarization. Rolipram or Ro-20-1724 reduced DSM spontaneous and carbachol-induced phasic contraction amplitude, muscle force, duration, and frequency, and electrical field stimulation-induced contraction amplitude, muscle force, and tone. Paxilline recovered DSM contractility, which was suppressed by pretreatment with PDE4 inhibitors. Rolipram had reduced inhibitory effects on DSM contractility in DSM strips pretreated with paxilline. This study revealed a novel cellular mechanism whereby pharmacological inhibition of PDE4 leads to suppression of guinea pig DSM contractility by increasing the frequency of Ca(2+) sparks and the functionally coupled TBKCs, consequently hyperpolarizing DSM cell MP. Collectively, this decreases the global intracellular Ca(2+) levels and DSM contractility in a BK channel-dependent manner.
Figures






Similar articles
-
Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 15;307(6):R571-84. doi: 10.1152/ajpregu.00142.2014. Epub 2014 Jul 2. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24990859 Free PMC article. Review.
-
Selective inhibition of phosphodiesterase 1 relaxes urinary bladder smooth muscle: role for ryanodine receptor-mediated BK channel activation.Am J Physiol Cell Physiol. 2012 Nov 15;303(10):C1079-89. doi: 10.1152/ajpcell.00162.2012. Epub 2012 Sep 19. Am J Physiol Cell Physiol. 2012. PMID: 22992675 Free PMC article.
-
Ethanol-mediated relaxation of guinea pig urinary bladder smooth muscle: involvement of BK and L-type Ca2+ channels.Am J Physiol Cell Physiol. 2014 Jan 1;306(1):C45-58. doi: 10.1152/ajpcell.00047.2013. Epub 2013 Oct 23. Am J Physiol Cell Physiol. 2014. PMID: 24153429 Free PMC article.
-
Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage- and Ca²⁺-activated K⁺ channel.Am J Physiol Cell Physiol. 2012 May 1;302(9):C1361-70. doi: 10.1152/ajpcell.00432.2011. Epub 2012 Feb 8. Am J Physiol Cell Physiol. 2012. PMID: 22322973 Free PMC article.
-
Prostaglandin E2 excitatory effects on guinea pig urinary bladder smooth muscle: a novel regulatory mechanism mediated by large-conductance voltage- and Ca2+-activated K+ channels.Eur J Pharmacol. 2014 Sep 5;738:179-85. doi: 10.1016/j.ejphar.2014.05.042. Epub 2014 Jun 2. Eur J Pharmacol. 2014. PMID: 24886877 Free PMC article.
Cited by
-
Novel mechanism of hydrogen sulfide-induced guinea pig urinary bladder smooth muscle contraction: role of BK channels and cholinergic neurotransmission.Am J Physiol Cell Physiol. 2015 Jul 15;309(2):C107-16. doi: 10.1152/ajpcell.00021.2015. Epub 2015 May 6. Am J Physiol Cell Physiol. 2015. PMID: 25948731 Free PMC article.
-
Elucidation of Cellular Mechanisms That Regulate the Sustained Contraction and Relaxation of the Mammalian Iris.Invest Ophthalmol Vis Sci. 2020 Sep 1;61(11):5. doi: 10.1167/iovs.61.11.5. Invest Ophthalmol Vis Sci. 2020. PMID: 32882011 Free PMC article.
-
Phosphodiesterase Inhibitors Revert Axonal Dystrophy in Friedreich's Ataxia Mouse Model.Neurotherapeutics. 2019 Apr;16(2):432-449. doi: 10.1007/s13311-018-00706-z. Neurotherapeutics. 2019. PMID: 30761510 Free PMC article.
-
Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 15;307(6):R571-84. doi: 10.1152/ajpregu.00142.2014. Epub 2014 Jul 2. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24990859 Free PMC article. Review.
-
BK channel activators and their therapeutic perspectives.Front Physiol. 2014 Oct 9;5:389. doi: 10.3389/fphys.2014.00389. eCollection 2014. Front Physiol. 2014. PMID: 25346695 Free PMC article. Review.
References
-
- Bender AT, Beavo JA. (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520 - PubMed
-
- Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. (2008) β-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295:F1149–F1157 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

